AUCTORES
Globalize your Research
Review | DOI: https://doi.org/10.31579/2640-1053/123
North Manchester General Hospital, Department of Urology, Manchester, M8 5RB, United Kingdom.
*Corresponding Author: Anthony Kodzo-Grey Venyo, North Manchester General Hospital, Department of Urology, Manchester, M8 5RB, United Kingdom.
Citation: Anthony Kodzo-Grey Venyo. (2022) Large Cell Neuroendocrine Carcinoma of the Prostate Gland: A Review and Update. J. Cancer Research and Cellular Therapeutics. 6(4); DOI:10.31579/2640-1053/123
Copyright: © 2022 Anthony Kodzo-Grey Venyo, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 27 May 2022 | Accepted: 20 June 2022 | Published: 30 June 2022
Keywords: large cell neuroendocrine carcinoma of prostate gland; prostate biopsy; trans-urethral resection of prostate; prostatectomy; radiotherapy; chemotherapy; histopathology; immunohistochemistry; aggressive treatment; early diagnosis
It has been documented that the 2016 World Health Organization’s histological classification of prostate cancer has included well-differentiated carcinoid tumours and small- or large-cell poorly differentiated tumours within the category of neuroendocrine tumours. Up to May 2021, only 20 cases of large-cell neuroendocrine tumours of the prostate gland (LCNTPs) had been reported within the literature, among which are nine cases of primary tumours. LCNTPs are a rare histological entity whose evolutive profile and therapeutic potential do differ from those of conventional adenocarcinoma of the prostate gland. Primary neuroendocrine tumours of the prostate gland could be pure or associated with an adenocarcinoma component. Mixed forms of large cell neuroendocrine cancers of the prostate gland (LCNECPs) tend to associated with better prognosis when diagnosed early at a localized stage. Nevertheless, most cases of (LCNTPs) at the time of initial diagnosis could tend to be advanced, locally advanced or metastatic. Even though LCNECPs that are diagnosed initially tend to primary prostate cancers that may be pure primary prostate cancers, few large LCNECPs have been found to be metastatic large cell neuroendocrine carcinomas that had metastasised from other sites of the body for example the lung. Primary LCNECP does tend to manifest similarly to primary adenocarcinoma of prostate gland with lower urinary tract symptoms, haematuria, or signs of obstruction of the upper urinary tract or inability to empty the urinary bladder. Cases of primary LCNECP that manifest tend also to be associated with symptoms related to the sites of the metastases. Serum prostate specific antigen levels tend to be slightly elevated with cases of primary LCNECP, but the levels generally tend not to be as high as most cases of advanced, locally advanced or metastatic primary adenocarcinomas of the prostate gland. Diagnosis of LCNECPs tend to be made pursuant to the undertaking of histopathology and immunohistochemistry staining studies of specimens of the prostate that had been obtained from prostate biopsies, trans-urethral reception of prostate specimens or prostatectomy specimens. The microscopy histopathology examination features of LCNECP the prostate gland have been summarized as follows:
(a) Microscopy histopathology examination of LCNECP gland does tend to demonstrate large islands or sheets of tumour cells. (b) Large tumour cells with prominent neuroendocrine features such as salt and pepper chromatin and small nucleoli tend to be visualised upon microscopy examination of specimens of the prostate tumour. (c) High grade features such as lack of glandular formation, frequent mitoses and apoptotic bodies and tumour necrosis tend to be frequent findings upon microscopy examination of specimens of LCNECP.
Immunohistochemistry staining features of LCNECP include: (a) At least one of the ensuing neuroendocrine tumour markers should be demonstrated upon immunohistochemistry staining including exhibition of positive immunohistochemistry staining for: chromogranin A, synaptophysin, neuron specific enolase, and CD56 may be positive: It has been stated that immunohistochemistry staining studies may exhibit positive staining in cases of LCNECP with utilization of the following:
o TTFI tends to be positive in less than 50% of cases of LCNECP.
o Positive AMACR immunohistochemistry staining, but this may be focal or weaker than for adenocarcinoma of the prostate gland.
o Positive markers including: PSA, PSMA, NKX3.1 prostein (P501S) tend to be negative in majority of cases of LCNECP, but they could be focally positive in a small subset of the tumours.
It has been iterated that in cases of LCNECP, immunohistochemistry staining studies tend to demonstrate negative staining for the ensuing markers:
• Urothelial markers such as GATA3, p63, and high molecular weight cytokeratins such as CK5 / 6.
• CD99.
• Carcinoid tumour of the prostate gland.
• PNET/Ewing sarcoma of the prostate gland.
• Adenocarcinoma of the prostate gland with focal neuroendocrine differentiation.
• Urothelial carcinoma with neuroendocrine differentiation.
A high index of suspicion is required in order to diagnose early cases of primary large cell neuroendocrine carcinomas by undertaking early biopsies of prostate glands in all patients who have significant lower urinary tract symptoms if their serum prostate specific antigen (PSA) levels are slightly high or high even if they are commenced on Tamsulosin in an attempt to help improve the symptoms of voiding.
There is no consensus opinion on the best management of LCNECP, therefore, there is an urgent need for the establishment of a global multi-centre trial related to various management options for the disease in order to ascertain the best options of management for LCNECP. gland.
It has been iterated that large-cell neuroendocrine carcinoma (LCNEC) of the prostate gland is an exceptionally rare type of prostate cancer.[1] and that only eighteen case reports had been published in the literature up to 2020 [1-5]. To the knowledge of the author less than 25 cases of LCNEC had been reported in the literature and the reported cases have been about 20 cases. LCNEC of the prostate is stated to be very aggressive and associated with widespread metastases [1] [5] [6]. The commonly reported sites of metastasis had tended to be to the lymph nodes, lungs, bones, and visceral organs, especially the liver. [1, 5, 6] Brain metastasis of LCNEC of the prostate had only been reported in two cases that had been published by Evans et al. [5], but no neuroimaging, gross, and microscopic evaluation of the brain lesion had been published [1, 5]. Aljarba et al. [1] stated that up to the time of publication of their article and in line with the SCARE criteria, their reported case was the first case to be published in the literature to describe brain metastasis of LCNEC of the prostate gland with neuroimaging, gross, and microscopic evaluation with immunohistochemistry. [1] [7].The ensuing article entitled Large Cell neuroendocrine carcinoma of the prostate gland: A Review and Update has been divided into two parts: (A) Overview which contains general statements on various aspects of Large Cell neuroendocrine carcinoma of the prostate gland in order to provide a bird’s eye view of the malignancy and (B) Miscellaneous narrations and discussions related to some case reports, case series as well as studies related to Large Cell neuroendocrine carcinoma of the prostate gland in order to provide an update on Large Cell neuroendocrine carcinoma of the prostate gland.
Various internet data bases were searched including: Google; Google Scholar; Yahoo; and PubMed. The search words that were used included: Large Cell Neuroendocrine carcinoma of the prostate gland; Primary Large Cell Neuroendocrine carcinoma of the prostate gland; metastatic Large Cell Neuroendocrine carcinoma of the prostate gland; prostatic Large Cell Neuroendocrine carcinoma. Thirty eight references were identified which were used to write the article on large cell neuroendocrine carcinoma of the prostate gland which has been divided into two parts: (A) Overview which contains general statements on various aspects of Large Cell neuroendocrine carcinoma of the prostate gland in order to provide a bird’s eye view of the malignancy and (B) Miscellaneous narrations and discussions related to some case reports, case series as well as studies related to Large Cell neuroendocrine carcinoma of the prostate gland in order to provide an update on Large Cell neuroendocrine carcinoma of the prostate gland.
Overview
Definition / general statements
Large Cell neuroendocrine carcinoma of the prostate gland, is a terminology that is used for high grade prostatic carcinoma which is composed of large cells with diffuse neuroendocrine features. [8]
It has been iterated that neuro-endocrine differentiation of prostate carcinoma is classified as follows: [6] [9]
Usual prostatic adenocarcinoma which is associated with neuroendocrine differentiation
Prostatic adenocarcinoma which is associated with Paneth cell neuroendocrine differentiation
Carcinoid tumour
Small cell carcinoma
Large cell neuroendocrine carcinoma
Mixed (small or large cell) neuroendocrine carcinoma which contain the usual prostatic adenocarcinoma
Essential features
With regard to essential features, large Cell neuroendocrine carcinoma of the prostate gland is said to be composed of tumour cells that are larger than those in small cell (neuroendocrine) carcinoma, also which contain slightly more cytoplasm [8] [10] [11]; nevertheless, the size cut-off point has not been well established
It has also been iterated that Large Cell neuroendocrine carcinoma of the prostate gland is similar to small cell carcinoma [8] [12] with regards to the ensuing features:
high grade tumour, with no prominent glandular differentiation
diffuse tumour, but with no focal neuroendocrine features
the tumour contains high rates of mitosis and apoptosis
the tumour tends to be associated with poor prognosis
The tumour could be pure or mixed with conventional high grade prostatic adenocarcinoma, either acinar or ductal type
Within the tumour, Paraneoplastic syndromes such as Cushing syndrome (ACTH producing tumour induced) could be present but tend to be rare
Epidemiology
It has been iterated that the epidemiology of Large Cell neuroendocrine carcinoma of the prostate gland is the same as for prostatic adenocarcinoma [8]
Aetiology
The ensuing iterations have been made with regard to the aetiology of Large Cell neuroendocrine carcinoma of the prostate gland: [8]
The aetiology is not known
Large Cell neuroendocrine carcinoma of the prostate gland might occur after androgen deprivation therapy for prostate cancer. [8] [13] Nevertheless, many cases of Large Cell neuroendocrine carcinoma of the prostate gland do develop de novo or in association with untreated high grade prostatic adenocarcinoma [12]
Clinical features
Clinical features of Large Cell neuroendocrine carcinoma of the prostate gland, have been summated as follows: [8]
The clinical features of Large Cell neuroendocrine carcinoma of the prostate gland tend to be similar to that of adenocarcinoma of the prostate gland.
Even though the serum PSA level may be elevated, many cases of Large Cell neuroendocrine carcinoma of the prostate gland do not show significant elevations of serum PSA, particularly considering the high tumour volume
Other symptoms and signs of advanced prostate cancer could occur
Serum neuroendocrine markers such as chromogranin could be elevated in cases of Large Cell neuroendocrine carcinoma of the prostate gland.
Diagnosis [8]
With regard to the diagnosis of Large Cell neuroendocrine carcinoma of the prostate gland, it has been iterated that tissue diagnosis with histology is essential, either from needle core biopsy of the prostate lesion, transurethral resection of prostate specimen or prostatectomy specimen.
It has also been stated that Cytology diagnosis of a primary tumour is not generally accepted as an initial diagnosis, but this could be utilized for metastatic sites.
Biopsies of the prostate gland could be undertaken as trans-perineal template biopsies of the prostate gland, or by means of trans-rectal ultrasound-guided biopsies of the prostate gland, but because trans-rectal ultrasound-guided biopsies of the prostate gland would tend to be associated with more infections, trans-perineal biopsies of the prostate gland are being undertaken these days more often than trans-rectal ultrasound-guided biopsies of the prostate gland.
Laboratory Studies
Microbiology
Urinalysis, urine microscopy and culture.
Routine urinalysis, urine microscopy and culture tend to be undertaken in all patients as part of the general assessment of patients who have Large Cell neuroendocrine carcinoma of the prostate gland and if there is any evidence of urinary tract infection, it would be treated appropriately based upon the sensitivity pattern of the cultured organism in order to improve upon the general health state of the patients as part of their general management in addition to their specific management.
Haematology
Routine haematology blood tests including full blood count and INR, tend to be undertaken in all patients who have Large Cell neuroendocrine carcinoma of the prostate gland and those patients who are found to have anemia would undergo treatment to correct their anemia so as to improve upon their general state of health as part of their general management as well as they would undergo appropriate management of their malignant carcinoma.
Biochemistry
Routine biochemistry blood tests including serum urea, EGFR, CRP, blood glucose, liver function tests, and bone profile tend to be undertaken in all patients who have Large Cell neuroendocrine carcinoma of the prostate gland, as part of their general assessment and if there is any abnormality, it would be investigated further as well as treated to improve upon the general state of health of each patient. If there is evidence of impaired renal function and there is evidence of obstruction of the ureter by the carcinoma of the prostate gland based upon radiology imaging, for example based upon ultrasound scan of the renal tract then nephrostomy insertion plus /minus insertion of antegrade ureteric stent or cystoscopy and insertion of retrograde ureteric stent into the obstructed ureter would be undertaken to improve upon the renal function of the patient as part of the general management of each patient in addition to provision of appropriate treatment for the carcinoma. Serum prostate specific antigen (PSA) level in cases of large cell neuroendocrine carcinoma of the prostate gland would be raised but the levels may not be as high as in pure adenocarcinomas of the prostate gland.
Radiology Imaging
Chest X-ray
Chest X-ray tends to be undertaken as part of the general assessment of patients to ascertain if there is any obvious metastatic lesion within the lungs and this tends to be undertaken most often in a number of hospitals in the developing countries especially where facilities for computed tomography scan and magnetic resonance imaging scan are not generally available. Within the developed countries Computed tomography scan and magnetic resonance imaging scan which forms part of the staging as well as follow-up assessment radiology imaging has replaced the undertaking of routine chest X-ray.
Ultrasound scan
Ultrasound scan of renal tract, ultrasound scan of the prostate as well as trans-rectal ultrasound scan-guided biopsy of the prostate lesion tend to be undertaken in many centres. If there is evidence of obstruction of the ureter by the carcinoma of the prostate gland then insertion of nephrostomy into the obstructed upper renal tract plus / minus insertion of antegrade ureteric stent into the obstructed upper urinary tract or cystoscopy and insertion of retrograde ureteric stent into the obstructed upper renal tract to improve upon as well as maintain good renal function as part of the general management of each patient.
Computed Tomography (CT) scan
CT scan of thorax, abdomen and pelvis and prostate, tend to be undertaken as part of the general staging as well as follow-up of patients who have Large Cell neuroendocrine carcinoma of the prostate gland. Additionally, CT scan of the prostate gland that is undertaken initially would demonstrate abnormal areas within the prostate gland that could be targeted for biopsy of the prostate as part of their template biopsies of the prostate gland based upon the radiology imaging findings.
Magnetic Resonance Imaging (MRI) Imaging
MRI scan of thorax, abdomen and pelvis and prostate, tend to be undertaken as part of the general staging as well as follow-up of patients who have Large Cell neuroendocrine carcinoma of the prostate gland. Additionally, MRI scan of the prostate gland that is undertaken initially would demonstrate abnormal areas within the prostate gland that could be targeted for biopsy of the prostate as part of their template biopsies of the prostate gland based upon the radiology imaging findings.
Isotope Bone Scan
Isotope bone scan tends to be undertaken to ascertain if patients who have large cell neuroendocrine carcinoma of the prostate gland have or do not have bone metastasis in order to plan their further management.
Positron Emission Tomography / Computed Tomography Scan
Positron Emission Tomography / Computed Tomography Scan is a radiology imaging technique that tends to be utilized in the follow-up assessment of patients who have large cell neuroendocrine carcinoma of the prostate gland in order to identify early metastatic lesions and locally recurrent lesions.
Prognostic factors
The ensuing summations have been made regarding the prognostic factors of Large Cell neuroendocrine carcinoma of the prostate gland: [8]
Large Cell neuroendocrine carcinomas of the prostate gland, often tend to be advanced disease at the time of the initial diagnosis of the tumours.
Large Cell neuroendocrine carcinomas of the prostate gland, tend to be high volume disease and they tend to be associated with poorer prognosis
Treatment
The ensuing summations have been made regarding the treatment of Large Cell neuroendocrine carcinoma of the prostate gland: [8]
There is very limited treatment experience reported in the literature, because Large Cell neuroendocrine carcinoma of the prostate gland is rare and probably it has been underreported.
Large Cell neuroendocrine carcinoma of the prostate gland, generally, does require systemic therapy similar to small cell carcinoma, but may not respond well to this treatment or to that for conventional prostatic adenocarcinoma
Macroscopic description
It has been stated that gross examination of specimens of Large Cell neuroendocrine carcinoma of the prostate gland, usually tend to demonstrate large tumour nodule(s) with a high volume of disease within the prostate gland. [8]
Microscopic (histology examination) description
The microscopy histopathology examination features of Large Cell neuroendocrine carcinoma of the prostate gland have been summarized as follows: [8]
Microscopy histopathology examination of Large Cell neuroendocrine carcinoma of the prostate gland does tend to demonstrate large islands or sheets of tumour cells.
Large tumour cells with prominent neuroendocrine features such as salt and pepper chromatin and small nucleoli tend to be visualised upon microscopy examination of specimens of the prostate tumour.
High grade features such as lack of glandular formation, frequent mitoses and apoptotic bodies and tumour necrosis tend to be frequent findings upon microscopy examination of specimens of Large Cell neuroendocrine carcinoma of the prostate gland.
Immunohistochemistry staining features of Large Cell neuroendocrine carcinoma of the prostate gland
Positive Immunohistochemistry staining:
The positive immunohistochemistry staining which has tended to be found in cases of Large Cell neuroendocrine carcinoma of the prostate gland before a diagnosis is made have been summarized as follows: [8]
At least one of the ensuing neuroendocrine tumour markers should be demonstrated upon immunohistochemistry staining including exhibition of positive immunohistochemistry staining for: chromogranin A, synaptophysin, neuron specific enolase, CD56.
May be positive:
It has been stated that immunohistochemistry staining studies may exhibit positive staining in cases of Large Cell neuroendocrine carcinoma of the prostate gland with utilization of the following:
TTFI tends to be positive in less than 50% of cases of Large Cell neuroendocrine carcinoma of the prostate gland.
Positive AMACR immunohistochemistry staining, but may be focal or weaker than for adenocarcinoma of the prostate gland.
Positive markers including: PSA, PSMA, NKX3.1 prostein (P501S) tend to be negative in majority of cases of Large Cell neuroendocrine carcinoma of the prostate gland, but they could be focally positive in a small subset of the tumours.
Negative stains
It has been documented that in cases of Large Cell neuroendocrine carcinoma of the prostate gland, immunohistochemistry staining studies tend to demonstrate negative staining for the ensuing markers: [8]
Urothelial markers such as GATA3, p63, and high molecular weight cytokeratins such as CK5 / 6.
CD99.
Differential diagnosis
The differential diagnoses of Large Cell neuroendocrine carcinoma of the prostate gland have been iterated to include the ensuing: [8]
Carcinoid tumour of the prostate gland.
PNET/Ewing sarcoma of the prostate gland.
Adenocarcinoma of the prostate gland with focal neuroendocrine differentiation.
Urothelial carcinoma with neuroendocrine differentiation.
[B] Miscellaneous Narrations and Discussion from some case reports, case series, and studies related to Large Cell Neuroendocrine Carcinoma of the Prostate Gland.
Yang [14] reported a 69-year-old man who did not have any previous prostate cancer history and who had undergone prostate needle core biopsy. Large neuroendocrine carcinoma was detected in every tissue-cores from all six locations examined. One small focus of adenocarcinoma was identified. Immunohistochemistry staining of the tumour had confirmed that the tumour cells had exhibited positive staining for NSE, synaptophysin and chromogranin, and AMACR negative for PSA and high Ki67 proliferative activity. His disease progressed quickly after the patient declined chemotherapy and other treatments. A few months later, he was found to have multiple metastatic lesions within his bone, lymph nodes and internal organs. He had biopsy of a lymph node and pathology examination of the specimen confirmed the diagnosis of metastatic large neuroendocrine carcinoma of the prostate gland. The patient received radiotherapy for bone lesions, but he had a poor response to radiotherapy, which failed to control the progression of his disease. He was sent to hospice for palliative care 11 months after the initial diagnosis of his malignancy. Pathology examination of his tumour specimen showed Large neuroendocrine carcinoma of the prostate gland which was composed of large tumour cells with slightly more cytoplasm and fine “salt-pepper” chromatin. Chromogranin staining demonstrated strongly and diffusely positive staining within the tumour cells. Yang [4] summarized the pathology examination findings related to the case as follows:
Large neuroendocrine carcinoma of the prostate gland was found in a 69-year-old man, which was composed of sheets of large tumour cells with “salt and pepper” chromatin (A).
Coexisting adenocarcinoma of the prostate gland was also seen as a minor component.
The Neuroendocrine (NEC) tumour cells exhibited diffuse positive staining for chromogranin and AMACR, triple stain), but the tumour cells had exhibited negative staining for HMWCK and p63 and PSA.
The Ki67 proliferative index of the tumour was up to 50% of tumour cells.
Sleiman et al. [15] made the ensuing iterations:
The 2016 World Health Organization’s histological classification of prostate cancer has included well-differentiated carcinoid tumours and small- or large-cell poorly differentiated tumours within the category of neuroendocrine tumours [16]
Only 20 cases of large-cell neuroendocrine prostate tumours had been described in the literature, among which nine represented cases of primary tumours. [17]
Large-cell neuroendocrine prostate tumours are a rare histological entity whose evolutive profile and therapeutic potential does differ from those of conventional adenocarcinoma.
Primary prostate neuroendocrine tumours could be pure or associated with an adenocarcinoma component.
Mixed forms of the tumour had better prognosis when they were diagnosed early at a localized stage.
Sleiman et al. [15] reported a 68-year-old Caucasian patient who had manifested in September 2015 with an elevated serum prostate-specific antigen (PSA) level. The patient’s past medical history included diabetes mellitus, hypertension, dyslipidemia, hyperuricemia as well as mild renal failure. The patient’s serum PSA level was 6.67 ng/ml in February 2015 and 9.65 ng/ml in September 2015. Upon clinical examination, a medium-sized prostate was found, with a palpable induration of the left lobe of the prostate gland, which was clinically classified as a stage T2b cancer. Twelve ultrasound-scan-guided prostate core biopsies were undertaken in October 2015. All biopsied tissues from both lobes were observed to contain tumour which was infiltrated by a large-cell neuroendocrine carcinoma that was associated with acinar adenocarcinoma with Gleason score 7(3 + 4), with perineural invasion and which did not have any infiltration of the capsule of the prostate. Multiparametric magnetic resonance imaging scan of the prostate gland in December 2015 demonstrated a prostate gland which weighed 43 grams with a suspicious lesion within the posterior peripheral zone, between the apex and the mid-gland of the left lobe of the prostate, that was classified as Pi-Rads 5/5, without infiltration of seminal vesicles and without capsular invasion or pelvic adenopathy. A bone scan was undertaken in November 2015 which showed no bone secondaries.
Considering that the majority component revealed on pathology examination of the biopsies was the neuroendocrine component, a 18F-fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG PET/CT) was undertaken in November 2015, which showed a prostate metabolic hyper-fixation without suspicious distant fixation (see figure 1).
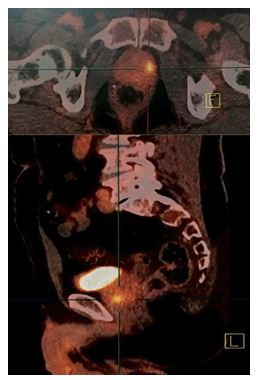
In view of the rarity of the histological entity of the tumour and the localized tumour character, Sleiman et al. [15] decided to undertake curative surgical treatment by radical prostatectomy with bilateral extended pelvic lymph node dissection. The Surgical operation was undertaken in January 2016. Operative exploration revealed that the prostate tumour had invaded the urinary bladder neck and trigone. Radical prostatectomy, bilateral pelvic lymph node dissection and partial cystectomy involving the bladder neck and trigone in macroscopically tumour-free limits and bilateral ureterovesical reimplantation were undertaken.
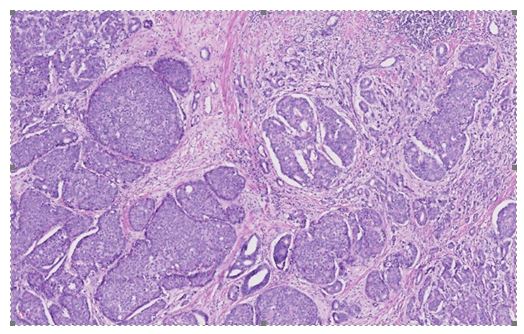
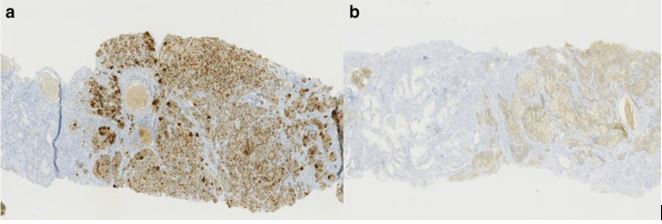
Based upon histology examination features of the tumour, Sleiman et al. [15] concluded that the tumour was a bilateral mixed prostate carcinoma which had invaded 80% of the prostate gland. The large-cell neuroendocrine component did comprise of 70% of the tumour and the acinar adenocarcinoma component amounted to the remaining 30% of the tumour. The Gleason score of the adenocarcinoma component of the tumour was 7(3 + 4). There was evidence of capsular infiltration of the tumour within the base of the right lobe of the prostate gland. The urinary bladder neck and seminal vesicles were found to be invaded as well as the neurovascular bands. There was also evidence of perineural invasion and vascular embolus (see figure 2). The large-cell neuroendocrine component was confirmed by immunohistochemistry staining studies, with utilization of synaptophysin and chromogranin A, with a strong proliferative activity, as Ki67 expression was at 80% (see figure 3).
All of the dissected lymph nodes upon the right side were found to be negative, and one lymph node of the seven taken on the left side was metastatic, with demonstration of invasion by the large-cell neuroendocrine component. The tumour was therefore classified as pT4 N1 (1/13) R1. The patient’s post-operative serum PSA level was 0.01 ng/ml. Pursuant to a multidisciplinary medical consultation, it was decided to treat the patient with chemotherapy (etoposide carboplatin [VP16]) as an adjuvant therapy, to be followed by radiotherapy of the prostatic bed and pelvis.
A [18F] FDG PET/CT scan was undertaken in September 2016 which demonstrated absence of any hyper-metabolic site.
Chemotherapy with etoposide carboplatin (VP16) was commenced in February 2016, which was then interrupted in May 2016 after the second cycle due to the fact that he developed renal toxicity.
[18F] FDG PET/CT scan was undertaken in June 2016, 6 months following the surgical operation, had demonstrated two left latero-aortic hypermetabolic lymph nodes despite a serum PSA of 0.01 ng/ml. The [18F] FDG PET/CT scan which was undertaken in August 2016, 4 months after the end of chemotherapy, demonstrated a significant decrease in the metabolic character of the left latero-aortic infra-centimetric lymph node and the absence of a second suspicious hypermetabolic lesion; his serum PSA was 0.01 ng/ml. Intensity-modulated radiation therapy (IMRT; 6 MV photons) of the prostatic bed was undertaken between November and December 2016, with 70 Gy, which was administered in 35 sessions.
The patient’s follow-up assessment was then planned based upon the serum PSA level for the adenocarcinoma component and the [18F] FDG PET/CT findings for the large-cell neuroendocrine component. In July 2017 the patient manifested with an elevated serum PSA of 2.75 ng/ml; in November 2017 the serum PSA level was 4. 77 ng/ml. The [18F]FDG PET/CT scan which was undertaken in November 2017 demonstrated bilateral supra-centimetric iliac lymph nodes, poorly hypermetabolic, which were more indicative of the adenocarcinoma component. Androgen deprivation treatment with the luteinizing hormone-releasing hormone (LHRH) agonist triptorelin was commenced in December 2017. The biological response was noted to be favourable; in that the serum PSA level was 0.01 ng/ml and his serum testosterone was 0.2 ng/ml 3 months following the first injection of triptorelin. He had [18F] FDG PET/CT scan in June 2018, 6 months after commencement of his androgen deprivation treatment, which indicated a complete metabolic response.
The patient’s follow-up was biological, with the undertaking of serum PSA measurement every 3 months for the adenocarcinoma component and metabolic imaging with [18F] FDG PET/CT scan, every 6 months for the large-cell neuroendocrine component. At the time of the report of his case, after 54 months of follow-up, the patient was reported to be still receiving androgen deprivation treatment with triptorelin with complete biological and metabolic response. At the last check-up follow-up assessment in March 2020, his serum PSA level was 0.01 ng/ml and his [18F] FDG PET/CT scan demonstrated a complete metabolic response.
Sleiman et al. [15] made the ensuing summating discussions:
The first descriptions of neuroendocrine system cells were noted to be relatively recent, and these had dated from the middle of the twentieth century.
Neuroendocrine cells tend to be usually present within the prostate tissue and increasing in number after puberty. Neuroendocrine cells tend to be less frequent within acini, from which conventional adenocarcinoma of the prostate could develop. Neuroendocrine cells do play a role with regard to the growth and secretory functions of prostatic epithelium [18] and neuroendocrine cells tend to be recognizable by the absence of androgen receptors and through labelling by certain immunohistochemical tumour markers, such as chromogranin as well as synaptophysin.
PSA negativity in immunohistochemistry studies of the tumour is a fundamental criterion of neuroendocrine cell recognition [19].
It is important to realise that a focal expression of PSA could be found within neuroendocrine tumours and that high-grade acinar adenocarcinoma could also express neuroendocrine markers, which thus enable differential diagnosis with large-cell neuroendocrine tumours to be made. [17]
The incidence of neuroendocrine cells in conventional prostatic adenocarcinoma did vary in the different studies which had histologically assessed primitive tumours and metastases [20]
Focal neuroendocrine features within adenocarcinoma of the prostate gland are correlated to high-grade and undifferentiated tumours. In view of the fact that adenocarcinomas and neuroendocrine cells could share hybrid immunohistochemical features in some cases, it has been iterated that it is important to take into consideration the classical morphological aspects of neuroendocrine tumour cells. Large neuroendocrine cancer cells usually tend to be in large nests with peripheral palisading [9]
Heterogeneity in defining the neuroendocrine status in prostate tumours could also be related to the increase in life expectancy and the awareness with regard to the detection of focal neuroendocrine foci within adenocarcinoma of the prostate gland. Treatments that are based upon androgen receptor signalling inhibition might also interfere with the detection of neuroendocrine features, as with regard to the case of metastases biopsy [21] [22]
Two mechanisms had been described related to the pathogenesis of prostate neuroendocrine tumours. The first mechanism which had been postulated, which is most frequent, is the trans-differentiation of an acinar adenocarcinoma under long-term hormone treatment, with loss of androgen receptors. Neuroendocrine manifestations of prostate cancer are increasing in view of the prolongation of survival and the utilization of new hormone treatments. The second postulated mechanism is a malignant transformation of neuroendocrine cells that are usually present within prostatic glands. [5]
This mechanism of de novo tumours has been supported by carcinogenesis animal models of prostate neuroendocrine cells [23]
Taking into consideration the limited number of published cases and series, the risk factors that predispose to the development of de novo neuroendocrine tumours had not yet been established [17]
The 2016 histological classification of tumours of the urinary and genital system had described three entities of prostate neuroendocrine carcinoma: neuroendocrine differentiation of adenocarcinoma, well differentiated neuroendocrine tumours or carcinoid tumours as well as poorly differentiated neuroendocrine small- or large-cell tumours. The recognition of neuroendocrine tumours rests, are based upon morphology, functional and immunohistochemical staining criteria. [16]
Neuroendocrine tumours do differ from adenocarcinoma based upon the absence of PSA secretion, resistance to hormone treatment, early metastasis and rapid progression [19] Small-cell tumours are by far more frequently encountered tumours in comparison with large-cell tumours which had remained exceptional. The coexistence of the two forms within the same tumour could be observed. [19]
Large-cell de novo tumours are rare or uncommon. The most recent review of the literature, which was published in 2019, had listed only about 20 cases, of which 17 were related to primitive prostate tumours, which had included seven de novo and eight following hormone treatment. Among the reported cases of large-cell de novo tumours, only three observations had reported mixed tumours with two components, neuroendocrine and adenocarcinoma components. These three reported cases had responded favourably to hormone treatment. [17]
De novo neuroendocrine tumours, which were either small or large cells, associated with an adenocarcinoma component did appear to have a better prognosis in comparison with pure forms because of a certain degree of hormone sensitivity. This notion or concept of androgen dependence or resistance does determine the difference regarding the prognosis between the two forms. [6]
In view of the PSA secretion, mixed forms of the tumour tend to be susceptible to early diagnosis at the localized stage, which thus does provide the possibility of curative treatment.
Pure forms of the tumour, not secreting PSA, had often been diagnosed at an advanced stage.
Xiang Tu et al. had reported three cases of de novo large-cell pure neuroendocrine tumours, which were diagnosed pursuant to trans-urethral resection of the prostate gland, with an unfavourable course under chemotherapy. [17] Evans et al. had reported a similar case with a tumour that was classified as stage pT3a after radical prostatectomy, with the patient manifesting with local and metastatic cerebral recurrence under adjuvant chemotherapy [5] [20]
Therapeutic possibilities for the management of these types of tumours are similar to those of lung large-cell neuroendocrine tumours and these essentially tend to involve chemotherapy given the resistance to hormone therapy. The prognosis usually has tended to be unfavourable in the locally advanced and metastatic stages, with a very limited survival. [3], [24]
Their reported case was diagnosed at a locally advanced stage following the undertaking of ultrasound-guided biopsies. Taking into consideration the rarity of the histological type and the moderate renal failure which had limited the possibilities of subsequent chemotherapy, they had opted for radical prostatectomy rather than radiotherapy. [18]F-FDG PET/CT follow-up was justified for a better detection of neuroendocrine cell metabolism, in comparison with choline. The favourable and complete response to hormone therapy, after ganglionic recurrence, could be explained by the presence of an adenocarcinoma component which had coexisted with the neuroendocrine component within the same tumour. Complete remission and relatively long survival in comparison with the previously published cases had strongly depended upon the early diagnosis, curative initial surgical treatment as well as regular radiology imaging during the follow-up which enabled an early detection and adapted treatment of lymph nodes recurrences.
Sleiman et al. [15] made the following conclusions:
Large-cell primary neuroendocrine carcinoma of the prostate gland is an aggressive as well as a rare histological entity.
Curative surgery as treatment for large-cell neuroendocrine carcinoma of the prostate gland, should be envisaged in localized and locally advanced forms.
Hormone resistance in the pure forms does limit the therapeutic arsenal for metastatic forms.
The association with a hormone-sensitive adenocarcinoma component does improve prognosis.
The development of nuclear radiology imaging modalities does enable a better follow-up and early diagnosis of recurrence, with substantial optimized care.
Evans et al. [5] stated the ensuing:
Neuroendocrine (NE) differentiation in prostate cancer tends typically to be identified by immunohistochemistry staining studies as single cells in conventional adenocarcinoma.
Prostatic NE tumours, such as carcinoid or small cell carcinoma, are rare / uncommon and large cell NE carcinoma (LCNEC) had been reported only in case reports.
Evans et al. [5] had identified 7 cases of LCNEC and had compiled their clinicopathologic characteristics. Evans et al. [5] reported that in 6 cases, there was a history of adenocarcinoma which was treated with hormone therapy for a mean of 2.4 years and with a treatment range of 2 years to 3 years. The remaining case of Evans et al. [5] was de novo LCNEC. LCNEC was incidentally diagnosed in palliative transurethral resection specimens in 5 cases. The mean age of the patients at the time of the diagnosis with LCNEC was 67 years and the ages had ranged between 43 years and 81 years. The LCNEC was reported to comprise of solid sheets and ribbons of cells that contained abundant pale to amphophilic cytoplasm, large nuclei with coarse chromatin and prominent nucleoli along with brisk mitotic activity and foci of necrosis. In 6 cases, there were foci of admixed adenocarcinoma, 4 of which had demonstrated hormone treatment effects. LCNEC upon immunohistochemistry staining studies had exhibited strongly positive staining for CD56, CD57, chromogranin A, synaptophysin, and P504S/alpha methylacyl CoA racemase. There was strong bcl-2 overexpression, expression of MIB1, and p53 in >50% of nuclei, focally positive staining for prostate specific antigen and prostatic acid phosphatase and negative androgen receptor staining. Follow-up data was available for 6 patients, all of who died with metastatic disease at a mean time of 7 months and this had ranged between 3 months and 12 months after platinum-based chemotherapy. Evans et al. [5] iterated that LCNEC of prostate is a distinct clinicopathologic entity which typically does manifests pursuant to long-term hormonal therapy for prostatic adenocarcinoma and likely does emanate through clonal progression under the selection pressure of therapy.
Alijarba et al. [1] reported a 79 years old man who had presented to the neurosurgery clinic complaining of a headache and dizziness with upper and lower limb weakness over the preceding 8 months and urinary incontinence over the preceding 2 months. The patient was known to have hypertension and diabetes and he also had a history of adenocarcinoma of prostate with lung metastasis. He had prostate biopsy and pathology examination of the specimen demonstrated high-grade adenocarcinoma of the prostate with a Gleason score (4 + 5 = 9), and he was treated by means of androgen deprivation therapy (ADT) nine years earlier. Two years preceding his manifestation, he had a follow-up bone scan and computed tomography (CT) scan of the chest, abdomen, and pelvis which had shown that his lung lesion had disappeared, and no other metastasis was found. Upon clinical examination, the patient was found to be alert and oriented to time, place, and person with GCS of 15/15, the pupils were equal and bilaterally reactive, and the power was 4/5 in both his upper and lower limbs. At the time of his admission, his total prostate-specific antigen (PSA) and free-PSA levels were 12.3 ng/mL and 1.8 ng/mL, respectively.
He had unenhanced computed tomography (CT) scan of the brain which demonstrated a large right frontal lobulated peripherally hyper-attenuating mass with punctuating foci of calcification and central hypodensity which was surrounded by vasogenic oedema, and it was reported to be causing a mass effect upon the right frontal horn with mild leftward midline shift (see new figure 4). His Contrast-enhanced CT scan of the chest, abdomen, and pelvis did not reveal any metastatic lesions. The lesion was removed by a modified pterional and orbital osteotomy approach. The macroscopic evaluation of the tumour demonstrated a grey-tan mass that measured 5.5 cm × 4.5 cm × 2 cm with a lobulated outer surface and an attached strap of dura mater. The serial sectioning of the mass demonstrated areas of necrosis. Histopathology examination of the specimen with haematoxylin and eosin staining revealed a monomorphic infiltrate of large cells that contained prominent nuclei and ill-defined cytoplasmic membrane, which were arranged in variable size nests with evident necrosis and a mitotic rate more than 4/10 high-power fields (see figure 5). Immunohistochemistry staining study (IHC) of the tumour showed that the tumour had exhibited positive reaction with EMA, Cam 5.2, synaptophysin, PSA and AMACAR antibodies and the tumour cells had exhibited negative staining for S100, TTF-1, CK7, CK20 and CDX2 (see figure 6). The histopathology examination features of the tumour and focal positivity for PSA, AMACR, and synaptophysin (NE marker) had supported the diagnosis of LCNEC of prostatic origin.
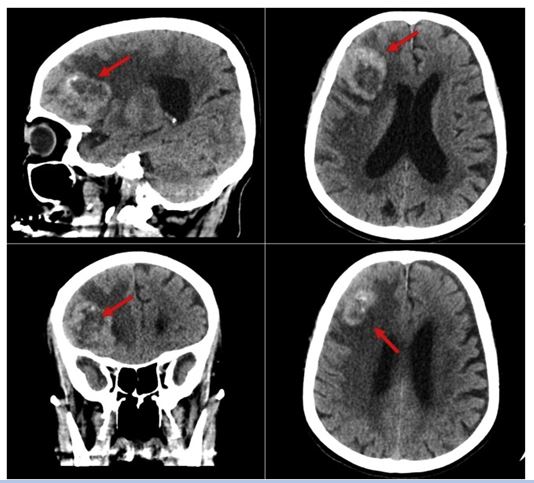


He had postoperative magnetic resonance imaging (MRI) scan which did not show any definite enhancing of a residual tumour with postoperative oedema and haemorrhage. Within the postoperative period, the patient did develop meningitis and minor surgical site infection, and his cerebrospinal fluid (CSF) analysis demonstrated low glucose levels and high protein levels with a negative CSF culture. On the fourth post-operative day, the patient did develop tachycardia, and an echocardiogram was undertaken which demonstrated severe aortic stenosis. In view of the age of the patient, and co-morbidities, no intervention was started, and the patient was commenced on aspirin 81 mg daily. On the fifth day pursuant to his surgery, the patient developed a decreased level of consciousness with GCS of 9/15, and he had a computed tomography (CT) scan which demonstrated dilated ventricles. In view of the fact that he had developed a raised intracranial pressure (ICP), an external ventricular drain (EVD) was inserted. He had a follow-up CT scan which demonstrated normal ventricular size, and his intracranial pressure (ICP) measurement was noted to be normal. Nevertheless, the patient’s neurological status did not improve. The patient was referred to the palliative care team for palliative care due to his multiple co-morbidities, and his poor clinical status. The patient died as a result of a cardiac arrest 43 days after his surgery.
Aljarba et al. [1] made the ensuing summating discussions:
The spectrum of neuroendocrine (NE) differentiation in carcinomas of the prostate gland could be classified based upon the 2016 WHO classification depending upon the pathophysiology and the molecular aspects of the disease. [6]
NE differentiation could be found as a focal differentiation within the usual acinar or ductal adenocarcinoma of the prostate gland which is identifiable by immunohistochemistry staining studies [6]
Carcinoid tumour of the prostate gland does exhibit well-differentiated NE tumour occurring within the prostate gland [6]
Small cell NE differentiation is a high-grade tumour of the prostate which had been defined by distinctive nuclear features such as the lack of prominent nucleoli, nuclear moulding, and crush artifacts [6]
It has been iterated that LCNEC is a high-grade NE tumour that is associated with distinctive morphological criteria of non–small cell carcinomas which does consist of large nests with peripheral palisading, large cell size, abundant cytoplasm, prominent nucleoli, vesicular clumpy chromatin, and frequent necrosis accompanied by a high mitotic rate and positive immunohistochemistry staining with at least one NE marker (synaptophysin, chromogranin, CD56) [6]
It has been documented that NE differentiation in carcinomas of the prostate gland tend to be very rare, and this does account for 1% to 5% of all cases of prostate cancer [5]
It had also been iterated that LCNEC is exceptionally rare in comparison with other NE tumours of the prostate gland, as well as it has been limited to sporadic case reports and case series [5] [6]
The largest case series was reported by Evans et al. in which the authors had discussed the pathological manifestations and the pattern of metastasis. [5].
It has additionally been documented that LCNEC could emanate from two possible pathological pathways. First, with regard to patients who had undergone treatment with utilization of long-term ADT for conventional adenocarcinomas in a process that is referred to as trans-differentiation. [25]
In vitro studies of the prostate cancer cell line LNCaP had demonstrated a reduction in androgen receptor expression in the cultures grown with the absence of androgens [25] This mechanism is said to be consistent with what is observable in some clinical cases including their reported case, in which there was a history of long-term ADT which had posed a selection pressure upon non-NE tumour cells from the conventional adenocarcinoma resulting in evolution and clonal proliferation and emergence of NE carcinomas with hormone-refractory status.
Castrate-resistance was observed in their reported patient, as his serum PSA level had risen and his brain metastasis had appeared, despite treatment.
Interestingly, it had been postulated that non-malignant NE cells of the prostate gland under adrenogenic depurative environment could promote androgen-independent growth of non-NE tumour cells in a paracrine fashion by secreting growth-promoting neuropeptides. [25].
It has been iterated that even though the evidence for LCNEC trans-differentiation had remained obscure, the existence of mixed NE carcinoma-acinar adenocarcinoma is one of the strongest-evidence of trans-differentiation. [5] [6].
It had been explained that another evidence is the presence of mixed features between the LCNEC and conventional adenocarcinoma such as co-expression of NE markers and PSA which does indicate the presence of intermediate forms of tumour cells and would further be in support of the process of trans-differentiation. [5]
Interestingly, their patient’s tumour IHC had exhibited focal positivity for PSA, AMACR, and synaptophysin which demonstrated mixed features between the LCNEC and conventional adenocarcinoma.
One of the most interesting results in animal models that also supported the process of trans-differentiation is probasin-large T antigen (Tag) transgenic mouse line which developed prostatic adenocarcinoma with progressive NE differentiation with advancing age, and metastasis that showed histological features and IHC of NE differentiation. [26]
Second, LCNEC could arise de novo by direct malignant transformation of NE cells of the prostate gland with no previous history of ADT. This mechanism was reported in a few case reports. [2] [3] [4] [5]
The relation between ADT and the progression of adenocarcinoma to NE carcinoma is still not well understood, and it does require further investigation.
It is understood that the development of NE differentiation, small cell or LCNEC type, does occur in a minority of patients and the spectrum of factors which play a role in the development of such an aggressive tumour is yet to be discovered.
Their patient had manifested with elevated levels of serum PSA.
Well-differentiated NE tumours (Carcinoid tumours) of the prostate gland do not express or secrete PSA [6]
Nevertheless; it was reported that LCNEC does express PSA variably ranging from complete negative staining to focal positivity with variable levels of serum PSA. [2] [5] [6]
This variability could be attributed to the presence of intermediate forms of cells that have mixed features between the LCNEC and conventional adenocarcinoma, the effect of treatment, and most importantly, the vague definition and poor understanding of LCNEC.
It has been documented that the pattern of metastasis of LCNEC does simulate the pattern that is observed in conventional prostatic adenocarcinoma demonstrating a preference for lymph nodes, lungs, bones, and liver [5].
Only two cases of brain metastasis from LCNEC had been previously reported in the literature by Evans et al. case series. [5]
Brain metastasis from NE tumours is extremely rare. The incidence of brain metastasis in a patient who is diagnosed with NE tumours was estimated to be between 1.5% to 5% and brain metastasis from NE tumours does account for 1.3% to 1.4% of all patients with brain metastasis [27]. Nevertheless; the pattern of metastasis and likelihood of metastasis in a patient with LCNEC of the prostate should be further investigated.
It is clear that the LCNEC of the prostate is a rare disease, and metastasis to the brain is also a rare event. Their patient had manifested with a headache and dizziness with upper and lower limb weakness with urinary incontinence and neuro-radiology-imaging had demonstrated a large right frontal mass. The brain mass was highly suspicious for metastasis and had raised other possible diagnoses such as high-grade meningioma or glioma. It has been iterated that Surgical excision of the tumour is the method of choice for large and symptomatic single brain metastasis which does provide quick symptomatic relief [28]. Post-operatively the patient had a tachycardia as a result of an unexpected severe aortic stenosis and this was diagnosed after the surgery. The patient did not tolerate the procedure very well, mainly due to his age and co-morbidities. The patient developed a decreased level of consciousness and dilated ventricles, and despite adequate treatment, the neurological status of the patient did not improve. It was decided not to resuscitate the patient in case he developed a cardiac arrest by signing the do not resuscitate (DNR) form and he was referred for palliative care. The patient survived for 43 days pursuant to his surgery.
Aljarba et al. [1] made the ensuing conclusions:
Patients’ complaints such as weakness, headache, altered consciousness, or focal deficits should be promptly investigated with detailed neurological history taking, physical examination, and neuroimaging.
The late diagnosis, the age of the patient, and the co-morbidities had worsened the prognosis of LCNEC of the prostate.
It is very clear that early detection and early treatment of metastatic LCNEC of the prostate gland would dramatically improve upon the outcomes as the metastasis and progression of NE trans-differentiation tend to be associated with the environment and age of the tumour.
Tzou et al. [3] stated that large cell neuroendocrine carcinoma (LCNEC) of the prostate gland is very rare and that previously reported cases of LCNEC in the literature were almost exclusively developed in men who had received androgen deprivation therapy for adenocarcinoma of the prostate gland. Tzou et al. [3] reported a case of de novo LCNEC: They reported a 66-year-old male who was incidentally diagnosed as having LCNEC after he had undergone transurethral resection of prostate. The stage of the tumour was T4N1M1. Therefore, the patient was treated with utilization of 6 cycles of cisplatin and etoposide in the ensuing 6 months, which achieved a partial remission. The chance of eradication of the residual mass had been given up. Three months subsequently, the tumour had progressed rapidly. Tzou et al. [3] made the following conclusions:
LCNEC is a rare carcinoma of the prostate gland.
Their experience had shown that chemotherapy with etoposide and cisplatin is effective to achieve a significant remission.
Nevertheless, LCNEC is highly malignant in nature, post-chemotherapy surgery for the residual mass should be considered as a treatment option.
Acosta-Gonzalez et al. [11] stated the ensuing:
Large cell neuroendocrine carcinoma of the prostate (LCNEC), de novo in particular, is an extremely rare clinical entity which had only been described in the literature in case reports.
Historically, the majority of the cases of LCNEC that had been reported in the literature had represented typical adenocarcinomas of the prostate gland which had transformed after long standing androgen deprivation therapy (ADT).
These cases were admixed with histological areas of usual adenocarcinoma and had shown hybrid features of both neuroendocrine and usual adenocarcinoma.
Acosta-Gonzalez et al. [11] reported a case of an LCNEC without admixed areas of the usual adenocarcinoma of prostate gland arising de novo in a patient without previous history of hormonal therapy. The tumour also showed morphological evidence of neuroendocrine differentiation; which had comprised of large sheets and nests of cells with moderate amphophilic cytoplasm with peripheral palisading, and vesicular clumpy chromatin with prominent nucleoli. The carcinoma’s prostatic origin was based upon positive immunohistochemical staining for PSA, PAP, PSMA, racemase, and Nkx3.1. Diffusely positive staining for chromogranin and synaptophysin, as well as the presence of secretory granules in the cytoplasm of the tumour cells was demonstrated by electron microscopy examination of the tumour which supported the NE differentiation. Acosta-Gonzalez et al. [11] stated that NE prostate cancer usually does not express upon immunohistochemistry staining of the tumour, AR and it is refractory to ADT therapy while AR and ERG were positive in their reported case.
Okoye et al. [24] stated the following:
Neuroendocrine (NE) differentiation in carcinomas of the prostate gland could be seen in two settings: as a focal finding in conventional acinar adenocarcinoma, identifiable by immunohistochemical staining, or as a primary NE tumour of the prostate gland, such as carcinoid tumour, small cell carcinoma, or large cell NE carcinoma of the prostate gland.
Of particular interest is the large cell NE carcinoma, that had been previously reported in isolated cases or in limited case series.
Okoye et al. [24] reported a case of a large cell NE carcinoma which was diagnosed in a 48-year-old man who had manifested with difficulty in voiding and urine retention. He underwent cystoscopy, which revealed an enlarged, elongated prostate gland with an intra-urethral obstructing mass within the prostatic urethra. Subsequently, a transurethral resection of prostate (TURP) was undertaken at an outside hospital under the clinical diagnosis of benign prostatic hyperplasia (BPH). Microscopy pathology examination of the TURP specimen demonstrated several foci of low-grade transitional-zone-type adenocarcinoma corresponding to Gleason score 5 (3 + 2), and a focus of high-grade large cell NE carcinoma. Concurrent x-ray computed tomography scans of the thorax, abdomen, and pelvis was undertaken which demonstrated an enlarged left pelvic lymph node, that was biopsied and the patient was diagnosed as having metastatic large cell NE carcinoma. He subsequently received 8 cycles of neoadjuvant chemotherapy with Lupron, as well as he underwent a laparoscopic robotic-assisted radical retropubic prostatectomy, and pelvic lymphadenectomy. He died of widely metastatic prostatic carcinoma with leptomeningeal metastases 13 months pursuant to his radical prostatectomy.
Miyakawa et al. [2] made the following iterations:
More than 95% of prostate cancers are adenocarcinoma, and neuroendocrine carcinomas (NECs) are very rare, which does account for less than 1% of prostate cancers.
Among NECs of the prostate gland including small cell carcinoma (SmCC), carcinoids, and large cell neuroendocrine carcinoma (LCNEC), LCNEC is very rare.
At the time of the report of their case, only 15 cases of LCNEC had been reported and their prognoses were very poor.
Miyakawa et al. [2] reported a patient who had a de novo LCNEC of the prostate gland which was successfully treated with the undertaking of radical surgery and adjuvant androgen deprivation therapy (ADT). Miyakawa et al. [2] reported an 87-year-old man, who was seen within their outpatient clinic with chief complaints of voiding difficulty and visible haematuria in August 2014. He had previously undergone left nephroureterectomy for a renal pelvic tumour in July 2010 as well as transurethral resection of the bladder tumour (TURBT) for a urinary bladder tumour in June 2014. The pathology examination findings of the tumour specimens were low-grade pTa urothelial carcinoma (UC) of the left renal pelvis and high-grade pTa UC of the urinary bladder, respectively. He underwent cystoscopy which revealed a sessile tumour on the neck of his urinary bladder, which was suspected as a muscle-invasive bladder cancer. He had computed tomography (CT) and magnetic resonance imaging (MRI) scans which demonstrated no evidence of metastases. His preoperative serum prostate-specific antigen (PSA) level was 3.3 ng/mL; nevertheless, the patient's prostate was found to be stony hard upon a digital rectal examination. In October 2014, he underwent TURBT and the pathology examination features of the tumour had indicated high-grade pT2 UC of the urinary bladder. In November 2014, he underwent radical cystoprostatectomy with urethrectomy, regional lymphadenectomy and right ureterocutaneostomy. The tumour was found to be located mainly within his prostate gland and partially within his urinary bladder. The left side of his prostate gland was firmly adherent to the pelvic wall, and it was difficult to peel off the site. Pathology examination of the tumour revealed features of LCNEC with microscopic focus of acinar adenocarcinoma, Gleason score of 2 + 3, of the prostate gland. The LCNEC consisted of large tumour cells which had high nucleus-to-cytoplasm (N/C) ratios, coarse nuclear chromatin, high mitotic rates, rosette structures, and fine granular cytoplasm. The tumour had replaced most of the prostate organ, which had confirmed the origin as prostatic, invading into the urinary bladder. Immunohistochemistry staining (IHS) studies of the LCNEC and adenocarcinoma had exhibited positivity for both PSA and androgen receptor (AR). Only the LCNEC exhibited positivity for CD56, chromogranin A, and synaptophysin. Two pathologists (KT and SM) had independently diagnosed the patient as having pT4 LCNEC and adenocarcinoma of the prostate with bladder invasion. A retrospective evaluation did reveal that the muscle-invasive part in the previous TURBT specimen was LCNEC. Even though was no evidence of lymph node metastasis, the tumour had a positive surgical margin, perineural invasion, and extracapsular invasion; therefore, Miyakawa et al. [2] started adjuvant ADT. Forty months pursuant to his diagnosis, the patient had survived with no evidence of tumour recurrence.
Miyakawa et al. [2] made the ensuing summating discussing iterations:
NECs are rare histological types of prostate cancer that tend to be associated with poor prognosis, and amongst them LCNEC is extremely rare.
Fifteen cases of LCNEC had been reported up to the time of the report of their case
Ten cases had occurred pursuant to long-term ADT, and five cases were de novo LCNEC. [4] [5] [11] [29]
The clinical features of 6 cases of de novo LCNEC including their reported case were illustrated as in Table 1.
Azad et al. [29] stated that ADT is likely effective for the treatment of de novo LCNEC because such tumours do remain androgen-dependency.
Out of five patients with de novo LCNEC whose prognoses were available in detail, three were alive without progression for more than 1 year.
It has been stated that even though LCNEC generated after long-term ADT has a miserable prognosis, [5] it is considered that de novo LCNEC has a relatively good prognosis.
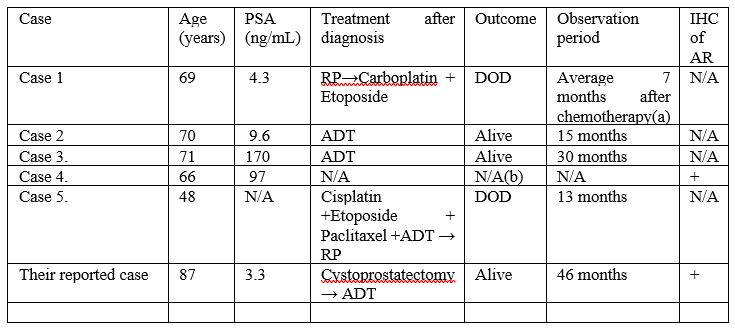
Table 1: Abbreviations: IHC, immunohistochemical staining; LCNEC, large cell neuroendocrine carcinoma; PSA, prostate specific antigen; AR, androgen receptor; RP, radical prostatectomy; DOD, died of disease; NA, not applicable; ADT, androgen deprivation therapy; (a) The observation period is described collectively with 6 cases, not respectively; (b) There is no description of outcome after diagnosis. Reproduced from: [2] under Creative Commons License
Immunohistochemistry staining studies (HIS) of AR was performed for two patients and both of them had exhibited positive staining for AR.
It has been stated that neuroendocrine cells, which commonly do exist in prostate tissue including prostate cancer, and NEC cells generally tend to exhibit negative staining for AR and they are considered to be androgen-independent. [30]
The expression of ARs on IHS does indicate androgen-dependency in hormone naïve prostate cancer.
AR-positivity of NEC also does indicate androgen-dependency and efficacy of ADT.
The long survival of their reported AR-positive and surgical margin-positive LCNEC of the prostate after adjuvant ADT does support the postulation.
Miyakawa et al. [2] made the following conclusions:
LCNEC of the prostate gland is extremely rare.
Majority of LCNEC cases present secondary to long-term ADT and the prognoses are generally very poor.
Their reported case had suggested effectiveness of ADT for androgen-dependent LCNEC of the prostate.
Androgen receptor-staining could be useful to predict efficacy of ADT on LCNEC of the prostate.
Aparichio et al. [31] made the following statements:
Small-cell carcinoma (SCC) of the prostate gland is an AR-negative variant of prostate cancer which is found at progression in 10–20% of castrate-resistant disease and its finding does predict a distinct clinical course and a poor prognosis.
Large-cell neuroendocrine carcinoma (LCNEC) is a much rarer variant which does tend to behave similarly to SCC.
The biological mechanisms which drive these disease variants are poorly understood.
With regard to the methods of their study, Aparichio et al. [31] stated that eight tumour fragments from the salvage pelvic exenteration specimen of a patient who had castrate-resistant prostate carcinoma were subcutaneously implanted into 6- to 8-week-old male CB17 SCID mice. Serial tissue sections and tissue microarrays of the resulting MDA PCa 144 xenograft lines were utilized for histopathology and immunohistochemical characterization of the xenografts and their tissue of origin. RNA from two representative xenograft sublines was used for gene-expression profiling. Aparichio et al. [31] summarized their results as follows:
All eight fragments formed tumours: four of the MDA PCa 144 xenograft sublines had morphology features of SCC and four, of LCNEC.
All retained high fidelity to their parent tumour tissue, which had remained stable through serial passages.
Morphological transitions within the specimen of origin had indicated that LCNEC does represent an intermediate step between adenocarcinoma and SCC.
Over 2,500 genes were differentially expressed between the SCC (MDA PCa 144-13) and the LCNEC (MDA PCa 144-4) sublines and enriched in “Nervous System Development” Gene Ontology subtree.
Aparichio et al. [31] made the ensuing conclusion:
The eight xenograft models that had been described do represent the spectrum of neuroendocrine carcinomas in prostate cancer and would be valuable preclinical tools to study the pathogenesis of and therapy targets for this increasingly recognized subset of lethal prostate cancer.
Yoo et al. [32] stated the following:
Large cell neuroendocrine carcinoma (LCNEC) of the lung is a rare and aggressive tumour that tends to be associated with a poor prognosis.
Lung cancer with metastases to the prostate gland are also uncommon, and they usually tend to be found incidentally during autopsy.
Majority of reported primary lung cancers with prostatic metastases are small cell carcinomas, and prostatic metastases from LCNEC of the lung had not been reported previously.
Yoo et al. [32] reported a 70-year-old man who had LCNEC of the lung and metastases in the prostate, brain, bone, liver and lymph nodes.
Zafarghandi et al. [4] reported a 71-year-old man who was referred to their clinic with increased dysuria, frequently and urgently for 3 months. He did not have any systemic signs and symptoms such as fever, night sweats, anorexia, and weight loss. Upon the digital rectal examination, his prostate gland was found to be enlarged. His serum Prostate specific antigen (PSA) level was found to be within the normal range (0.09 ng/mL). He had ultrasound scan which had indicated moderate enlargement of his prostate gland and Benign prostate hyperplasia (BPH). He was therefore given tamsulosin with regard to his BPH diagnosis. He was referred again without any relief after 3 months of undergoing Tamsulosin medication. His international prostate symptoms score (IPSS) was 22 before commencement of his treatment and it had remained unchanged after three months. He underwent transurethral resection of the prostate (TURP) because of the severity of his lower urinary tract symptoms and the patient’s desire to undergo surgical treatment to improve his symptoms. Histopathology examination of the resected prostatic chips and urine cytology was conducted after his TURP. The first pathologist had reported poorly differentiated carcinoma, so colonoscopy and whole-body scan were performed for him and both were negative. The second pathologist made a diagnosis of LCNEC. The patient refused to undergo treatment in this step and he re-manifested again after three months with uraemia, confusion, and poor general condition. He did complain of having sever pelvic pain. In the first instance, he had ultrasound scan of abdomen and pelvis which demonstrated bilateral hydronephrosis. He underwent haemodialysis due to his increased serum creatinine level (8 mg/dL) and ultrasound guided bilateral nephrostomy for obstructive uropathy. He subsequently had non-contrast CT scan which confirmed presence of a large pelvic mass with pelvic lymphadenopathy (see figure 7). His tumour was unresectable and he was referred to the oncology department to be considered for palliative radiotherapy. The haematoxylin and eosin slides of his TURP specimen demonstrated sheets and a large nest of high-grade neoplasm was found to be peripheral palisading, and diffusely infiltrating the prostate parenchyma. The neoplastic cells were typified by large vesicular nuclease which contained course chromatin and prominent nucleoli as well as abundant amphophilic cytoplasmic. There were also seen areas of geographic necrosis and high mitotic activity (see figure 8). These features in the reported specimen were reported to simulate the histological description of LCNEC reported by Evans et al. [5] in the largest series that had been published on LCNEC. Conventional adenocarcinoma was not found throughout the specimen. Immunohistochemistry (IHC) staining studies of the tumour was undertaken to ascertain the origin of the tumour and the extent of NE differentiation (see figure 8). IHC was found to be negative for PSA but the tumour exhibited positive staining for AMACR. In order to exclude carcinoma of the urinary bladder, IHC was undertaken for P63, CK20, CK 7, as well as for GATA-3. IHC staining with neuroendocrine (NE markers) including chromogranin, synaptophysin, and CD 56 was undertaken in order to establish NE differentiation. The results of the study demonstrated that all of NE markers were strongly and diffusely positive with cytoplasmic staining (see figure 9).
Zafarghandi et al. [4] made the ensuing summating iterations:
Large cell carcinoma with neuroendocrine differentiation is a-well known tumour and there are reports of it having been sporadically reported to be found with various origins including the lung, cervix, and larynx, (see Table 2).
These cancers had been stated to constitute a distinct pathological entity and to be associated with an unfavourable prognosis [33] [34]
LCNEC is a very uncommon malignancy in the prostate gland.
It has been stated that NePCs’s typical manifestation is symptoms that tend to be related to enlargement of the prostate gland [29]
Immunohistochemistry staining studies for Neuroendocrine markers such as synaptophysin, CD56, and chromogranin tend to exhibit positive staining in LCNEC.
It has been iterated that the PSA level does tend not to correlate with LCNEC signs and symptoms [33].
LCNEC had been reported in 7 patients in 2006 from Canada [5]. Out of these reported cases, only one of these patients was found to have de novo LCNEC and he was 69 years old. The tumour was diagnosed incidentally in 5 cases following palliative transurethral prostatectomy [34]. The serum PSA level in this patient was lower than 0.1 ng/mL like in their patient.
Their reported patient was not suspected of having NePCs in the first steps and palliative TURP was undertaken to relieve his symptoms.
In a case that was reported in 2014, a patient was evaluated for increased serum PSA level and he was finally diagnosed as having LCNEC [11].
Pelvic mass with rapid progression had previously been reported in some cases. [5]
In their reported case, huge pelvic mass had caused obstructive uropathy and worsened the patient’s clinical condition.
The mean survival time of NePCs had been estimated to be less than 12 months and this had ranged between 3 months and 12 months.
Their reported patient was alive during his 6 months follow-up.
Death does tend to occur as a result of metastasis and uropathy, and because of LCNEC delayed diagnosis, majority of cases of LCNEC tend to have metastatic disease.
Their reported patient, also, had metastasis.
Even though androgen-deprivation therapy (ADT) had been regarded as the main predisposing factor associated with NePCs, some reported cases did not have any positive point in their past medical history [35]
Okoye et al. [24] had reported a 48-year-old man who had LCNEC and no history of ADT.
A total of 6 cases that had been reported in Evans’s study [5] in which the patients had a history of ADT for adenocarcinoma of the prostate gland; nevertheless, their reported case did not have any predisposing factor.
LCNEC could manifest in young males and it might have a genetic base.
Animal model studies had demonstrated that prostate neuroendocrine cells could show a malignant transformation, as well.
Some de novo LCNEC cases do express androgen receptor (AR) and they might be androgen dependent but other cases of de novo LCNEC are AR negative [11].
The main and exact mechanism and underlying causes of LCNEC are not known.
In their reported case, CD56 was positive and Evans et al. [5] had shown that this immunohistochemical marker was positive in all LCNEC patients
It had been reported in previous studies that LCNEC cells do exhibit positive immunohistochemistry staining for: CD56, chromogranin, and synaptophysin.
LCNEC could be diagnosed if one of the markers become positive.
The first report of LCNEC Immunohistochemistry findings was reported by Wynn et al. in 2000 [36].
And this marker needs to be evaluated in suspected cases of LCNEC.
Travis et al. [37] did describe LCNEC by their specific immunohistochemistry (IHC) and electron microscopy (EM) features. They studied 5 cases of LCNEC and did show that these patients’ prognosis had varied between atypical carcinoid and small cell carcinoma.
The overall survival of patients who had NePCs had been estimated to range between 9 months and 12 months [5].
All patients that had been reported in the study of Evans et al. [5] died soon after diagnosis.
It had been stated that it did seem that increased neuroendocrine differentiation is correlated with more aggressive forms of diseases and a poor prognosis [5].
Their reported patient had been discharged from the hospital and 6 months pursuant to his discharge he was still alive.
Their reported patient did have severe pelvic pain which could have occurred as a result of neural invasion, or the compression effect of large pelvic lymph nodes as well as a large tumour.
In other case reports, large mass had led to urinary retention [24].
With regard to majority of cases, LCNEC had been diagnosed with delay or as late cases, and the tumour was not resectable.
Pelvic lymph node infiltration and metastases had tended to be common in patients who had LCNEC like in their reported patient.
LCNEC had responded poorly to standard NePCs chemotherapy protocols.
There are some recommendations for the utilization of novel and additional treatment such as somatostatin analogues in these cases, but it has been recommended that more cases need to be evaluated in order to develop the exact treatment strategy for LCNEC [6] [37] [38]
Zafarghandi et al. [4] made the ensuing conclusions:
Taking into consideration the fact that LCNEC as a differential diagnosis in patients with prostate cancer is important.
Even though at times large cell neuroendocrine carcinoma in prostate manifests as metastases from other organs like lung, it is important to take note of the occurrence of primary large cell neuroendocrine carcinoma of the prostate gland.
On the other hand, careful histological and immunohistochemistry (IHC) staining study examination of enlarged prostate with normal PSA level need to be determined in suspected cases, because it does influence the prognosis and designation of the therapeutic strategy of the patients.

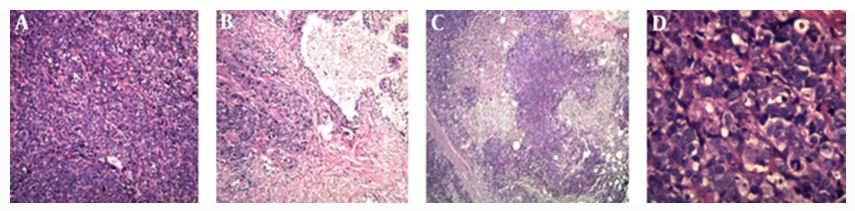
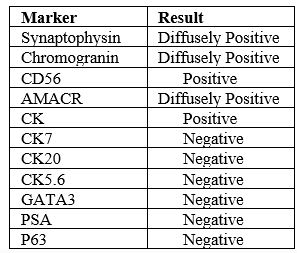
Table 2: Abbreviations: AMACR, alpha-methylacylcoA racemase; CD, cluster differentiation; CK, cytokeratin; GATA3, GATAbinding protein; PSA, prostatic specific antigen.

Priemer et al. [6] made the following iterations:
Neuroendocrine neoplasms of the prostate gland do represent a multifarious group of tumours which do exist both in pure forms and in association with adenocarcinoma of the prostate gland.
Morphologically, neuroendocrine cells within prostate neoplasms could range from being indistinguishable from encompassing adenocarcinoma of prostate cells to having high-grade neuroendocrine appearances similar to neuroendocrine malignancies of other organs.
On the molecular level, neuroendocrine malignancies which do arise in the setting of adenocarcinoma of the prostate gland had been the subject of a large amount of recent research, majority of which had supported the conclusion that neuroendocrine malignancy within the prostate does develop as a trans-differentiation from adenocarcinoma of the prostate gland.
There had not; nevertheless, been substantial investigation into rare, pure neuroendocrine malignancies and the possibility that these tumours could have a different cell of origin and molecular genesis.
They had discussed the morphological spectrum of malignant neuroendocrine prostate neoplasms and they had reviewed the most recent molecular data on the subject of malignant neuroendocrine differentiation in prostatic adenocarcinoma.
Upon reflection of the most recent data, they had also discussed the diagnostic classification of prostate neuroendocrine tumours with reference to the 2016 World Health Organization (WHO) classification.
They had discussed the reporting of these tumours, placing emphasis upon the differentiation between pure and mixed neuroendocrine malignancies in order that, in the least, they could be easily identified for the purposes of future clinical and laboratory-based investigation.
They had finally, suggested a designation for an unclassifiable (or not otherwise specified) high-grade neuroendocrine prostate malignancy whose features do not easily place it into one of the WHO diagnostic entities.
Tu et al. [17] iterated that large cell neuroendocrine carcinoma (LCNEC) of the prostate gland is an extremely rare entity, and the clinicopathological course, potential effective treatment, as well as prognosis of the tumour are yet to be elucidated. Tu et al. [17] undertook a systematic search utilizing various internet data search bases from the inception of large cell neuroendocrine cancer of the prostate gland to January 2019. Tu et al. [17] studied each individual case of LCNEC of the prostate gland, and they summarized specific features and outcomes for this uncommon pathology entity. Tu et al. [17] summarized the results as follows:
Thirteen studies with a total of 20 patients whose mean age was 70.3 years and whose ages had ranged between 43 years and 87 years were included in their review.
Seventeen patients had primary LCNEC of the prostate gland, of which 9 patients were diagnosed as having de novo carcinoma, and 8 patients had a history of adenocarcinoma of the prostate gland which had been treated by means of hormonal therapy over a mean duration time of 2.9 years and follow-up which had ranged between 2 years and 5 years.
The other 3 patients were diagnosed as having metastatic LCNEC which had originated from the lung in 2 cases, and the urinary bladder in 1 case.
All of the patients met the diagnostic criteria of the typical morphology characteristic features as well as immunohistochemistry staining features of large cell neuroendocrine carcinoma.
Nearly all of the primary de novo LCNEC of the prostate gland were at a late stage at the time of their initial diagnosis.
The pattern of distant metastasis simulated that of adenocarcinoma of the prostate gland with the commonest site as bone spread which was noted in 8 out of 16 cases that amounted to 50% of the cases.
Majority of the patients had undergone systematic chemotherapy after initial diagnosis; nevertheless, the prognosis of the patients had remained poor and the patients’ condition deteriorated rapidly but with exception.
Three reported cases in the context of de novo LCNEC which were admixed with adenocarcinoma of the prostate gland, kept sustained response to androgen deprivation therapy (ADT) and they obviously achieved superior / better survival outcomes in comparison with other patients.
Tu et al. [17] made the ensuing conclusions:
LCNEC of the prostate gland is a rare entity which mostly does occur pursuant to long-standing hormonal therapy of adenocarcinoma of the prostate gland.
The prognosis of LCNEC was universally poor irrespective of the systematic chemotherapy the patients received.
Nevertheless, patients who have de novo tumour that is mixed with adenocarcinoma of the prostate gland may respond to ADT and they may have a better outcome in comparison with those patients who have pure de novo or post-ADT LCNEC of the prostate gland.
Considering that globally the most common malignant tumour of the prostate gland that has been diagnosed is adenocarcinoma of the gland, there is the possibility that when some pathologists examine specimens of the prostate in which they find adenocarcinoma of the prostate gland, they may not extensively and critically examine every specimen and all areas of the prostate and they would establish a diagnosis of pure adenocarcinoma. However, perhaps if globally all pathologists thoroughly examine all areas of prostate specimens they receive, they may encounter mixed tumours including the possibility of LCNECP which they could report that would increase knowledge about the possibility of LCNECP being associated with adenocarcinoma of the prostate gland and if mixed tumours of the prostate gland including adenocarcinoma of the prostate and LCNECP is found the biological behaviour of mixed adenocarcinoma and LCNECP would be studied and reported. Additionally, if the immunohistochemistry staining antibodies that are utilized for the diagnosis of LCNECP are routinely used during the pathologist's examination of specimens of prostate cancer, perhaps more cases of LCNECP would be diagnosed globally Furthermore, if pathologists do not consider the possibility of LCNECP by undertaking a thorough examination of their prostate specimens, they would miss the diagnosis of LCNECP which does tend to portend a more aggressive biological behaviour and which does need an aggressive treatment in order to ensure good prognosis.
Large-cell primary neuroendocrine carcinoma of the prostate gland is an aggressive and a rare histological entity which could perhaps be due to under reporting of this type of prostate cancer or difficulties in establishing the diagnosis of this type of cancer by pathologists globally but one cannot be sure of this. Less than 25 cases of large cell endocrine carcinoma have been reported in the literature.
Curative surgery should be undertaken with regard to localized and locally advanced forms of primary large cell neuro-endocrine carcinoma of the prostate gland in view of the reported aggressive biological behaviour of the tumour.
Nevertheless, resistance in the pure forms of primary large cell neuroendocrine carcinomas (LCNECs) of the prostate gland does limit the treatment arsenal for metastatic forms of LCECs of the prostate gland.
The association of primary LCNEC of the prostate gland with a hormone-sensitive adenocarcinoma of prostate component does tend to improve upon the prognosis.
The development of nuclear imaging modalities does allow a better follow-up and early diagnosis of recurrence, with substantial optimized care.
A high index of suspicion is required in order to diagnose early cases of primary large cell neuroendocrine carcinomas by undertaking early biopsies of prostate glands in all patients who have significant lower urinary tract symptoms if their serum prostate specific antigen (PSA) levels are slightly high or high even if they are commenced on Tamsulosin in an attempt to help improve the symptoms of voiding.
Considering that there is no consensus opinion on the best management of large cell neuroendocrine carcinoma of the prostate gland, there is an urgent need for the establishment of a global multi-centre trial related to various management options for the disease in order to ascertain the best management of large cell neuroendocrine carcinoma of the prostate gland.
Urologists, oncologists, and pharmacists globally should endeavour to undertake research that would identify safe and effective chemotherapy medicaments that would help improve upon the prognosis of large cell neuroendocrine carcinomas of the prostate gland as well as other primary large cell neuroendocrine carcinomas of various sites of the body.
None
Acknowledgements to:
[1] Journal of Medical Case Reports for granting permission for reproduction of contents and figures from their journal article under copy right permission: Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third-party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
[2] International Journal of Surgery Case Reports for granting permission for reproduction of contents and figures of their journal article under copy right: Creative Commons
This is an open access article distributed under the terms of the Creative Commons CC-BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. You are not required to obtain permission to reuse this article.
[3] Urology. Case Reports for granting permission for reproduction of contents of their journal article including tables and figures under copy right © 2018 The Authors
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
[4] Nepho-Urology Monthly for granting permission for reproduction of contents, of their journal article including figures and tables under: Copyright © 2017, Nephology and Urology Research Centre. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Licence (http://creativecommons.org/licences/by-nc/4.0/), which permits copy and redistribute the material just in non-commercial usages, provided the original work is properly cited.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.
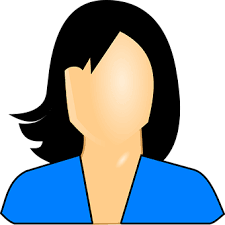
Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
