AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2640-1053/122
1 Department of Biochemistry, Cancer Biology, Neuroscience & Pharmacology, Meharry Medical College, Nashville, TN 37208, USA.
2 Department of Professional Education, Meharry Medical College, Nashville, TN 37208, USA.
*Corresponding Author: Salil K. Das, Sc.D., D.Sc., Professor of Biochemistry Cancer Biology, Neuroscience & Pharmacology, Meharry Medical College, Nashville, TN 37208, USA. Tel: 615 327-6988; Fax: 615 327-6442.
Citation: Shyamali Mukherjee, Sutapa Mukhopadhyay and Salil K. Das. (2022). Inhibition of Dimethylbenz[a]anthracene-induced Breast Tumors in Rats by Soy Protein is Mediated by Downregulation of MAPK/AP-1 Signaling. J Cancer Research and Cellular Therapeutics, 6(4); DOI: 10.31579/2640-1053/122
Copyright: © 2022, Salil K. Das. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 24 May 2022 | Accepted: 01 June 2022 | Published: 30 June 2022
Keywords: soy protein; casein; casein plus soy protein; breast cancer; MAPK and AP-1 signaling; proliferation; PCNA; Cyclin D1; uPA; MMP-9
We reported earlier that replacement of casein by soy protein in the diets of rats exposed to the carcinogen, dimethylbenz[a]anthracene (DMBA), not only delayed the initiation of breast tumor but also had a protective effect against the development of aggressive tumor. The aim of this study was to elucidate the molecular mechanism by which soy protein offers these beneficial effects. Tumor was developed by gavage administration of single dose of 80 mg/kg of DMBA into 50-day old female rats, maintained on a standard AIN-76A diet containing either casein or soy protein. After ~120 days of DMBA administration, we evaluated the role of MAPK phosphorylation and subsequent AP-1 activation on the differential effects of soy protein and casein on the development of aggressiveness and progression of DMBA-induced breast tumor and determined if soy protein controls MMP-9 and uPAR expression by modifying AP-1 activity. The present study demonstrates that the beneficial effect of soy protein in breast tumor development is mediated by control of MAPK/AP-1 signaling. It is concluded that deactivation of MAPK pathway lead to down-regulation of the AP-1 activation which in turn down regulates the target gene and may be responsible for controlling breast tumor aggressiveness. Thus, it is suggested that MAPKs (ERK, p38 and JNK), MMP-9 and uPAR may be a potential target for anticancer therapy inhibiting tumor vasculature and invasion stimulated by tumor-associated stroma, and regulating the target gene and may be responsible for the beneficial effects of soy protein.
Breast cancer is the second most common cancer globally and one of the leading cancers in women in the world and its incidence gradually increases year by year according to the world Health Organization [1, 2]. Etiology of breast cancer is composed of many factors including age, genetic, reproductive, hormonal, environmental factors and lifestyle such as cigarette smoking, alcohol and diet [3-6]. Several recent reports indicate that soy proteins either as intact soy protein or functional or bioactive peptides derived from soybean processing have a variety of physiological functions such as immunomodulatory effects, hypolipidemic, anti-inflammatory, antioxidant, anti-hypertensive, antiangiogenesis, anti-cancer properties [7-12]. Observational studies show that among Asian women higher soy consumption is associated with an approximately 30% reduction in risk of the developing breast cancer. Furthermore, Clinical trials and epidemiologic studies involving over 11,000 women from USA and China show that post-diagnosis soy intake statistically significantly reduces recurrence and improves survival [13-15]. Recent research also suggested that women who are at increased risk of breast cancer due to polymorphisms in genes associated with disease may especially benefit from high soy isoflavones intakes [16]. Furthermore, soy foods have long been recommended in the diets for breast cancer survivors and women at high risk for breast cancers [17]. Recent epidemiology evidence on the association of soy intake with breast cancer risk is still inconsistent due to different soy intake levels and or type of soy food such as whole soy, soy isoflavones, soy protein isolate [18-22].
Our previous study indicated that replacement of casein by soy protein in the diets of rats exposed to the carcinogen, dimethylbenz[a]anthracene (DMBA), not only delays the initiation of breast tumor development but also has a protective effect on the development of aggressive tumor [23]. While 100% of the breast tumor induced in rats fed soy protein was of grade I (non-aggressive) type, casein consumption produced more aggressive tumors (20% grade I, 60% grade II and 20% grade III). The mechanism by which soy protein regulates the development of aggressiveness and progression of DMBA-induced breast tumors remains to be elucidated. However, our subsequent study also indicated that replacement of casein by soy protein in diet suppresses the expression of a tumor-promoting gene, translocator protein (TSPO) in breast tumors in rats [24].
The mitogen-activated protein kinase (MAPK) cascades are multi-functional signaling pathways that transduces signals from the cell membrane to the nucleus in response to variety of stimuli and participates in various intracellular signaling pathways that control a wide spectrum of cellular processes, including proliferation, growth, differentiation, development, inflammatory response and stress responses, and is known to have a key role in cancer progression. It is a critical pathway for human cancer cell survival, dissemination, and resistance to drug therapy [25]. Three MAP kinase (MAPKs) cascades, such as extracellular signal-regulating kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 MAP kinases have been characterized [26, 27]. MAPKs are proline-directed serine/threonine kinases that are activated by dual phosphorylation on threonine and serine residues in response to a wide variety of extracellular stimuli [28, 29]. They mediate signal transduction from the cell surface to the nucleus. The ERKs family consist mainly of a kinase domain (ERK1 and ERK2) and is activated by mitogenic stimuli such as growth factors, cytokines, and phorbol esters and plays a major role in regulating cell growth, survival, and differentiation. The JNK and p38 MAPK are weakly activated by growth factors but respond strongly to stress signals including environmental stress, tumor necrosis factor (TNF), interleukin-1, ionizing and ultraviolet radiation, hyperosmotic stress, and chemotherapeutic drugs and activation of this pathway is strongly associated with inflammation, apoptosis, cell differentiation and cell regulation. A number of studies have reported that the JNK signaling pathway acting via c-Jun or the related transcription factor ATF-2 is involved in the modulation of DNA repair and/or cell survival in response to various forms of genotoxic damage [30-32]. MAPKs regulate AP-1 both at the transcriptional and post-translational levels, affecting its transactivation potential, DNA binding capacity and stability. Several studies reported that MAPK signaling pathways were key upstream of activator protein-1 (AP-1) and activation of MAPK regulates the expression level of AP- through the substrate phosphorylation and by influence on the abundance of individual AP-1 components in cells [33]. AP-1 is an important regulatory protein involved in various physiological and pathological cellular processes including cell proliferation, growth, migration, differentiation, transformation, and apoptosis [34]. The AP-1 complex consists of homo- or hetero- dimers of Fos families (c-Fos, Fos B, Fra-1 and Fra-2), activating transcription factor families (ATF-1,ATF-2 B-ATF, JDP1and JDP2) Jun subfamilies (c-Jun, Jun B and Jun D), and MAF (c-Maf, MafB, MafA and MafG/F/K) protein families that are characterized by highly conserved dimeric basic-region leucine-zipper (bZIP) DNA-binding sequences called 12-O-tetradecanoyl-phorbol-13 acetate (TPA) responsive elements (TRE, 5’-TGAG/CTCA-3’ or cAMP response elements, CRE, 5’-TGACGTCA-3’) [35]. The activity of this transcription-factor complex is modulated by growth factors, cytokines and tumor promoters [36]. The activated AP-1 dimer binds to specific DNA sequences in the regulatory regions of mitogen-responsive genes, several of which are involved in cellular processes such as proliferation or tumor invasion [37].
In breast cancer cells, the AP-1 proteins have been identified as important regulators of growth and invasion. As a member of the AP-1
transcription factor complex, c-Fos is an essential modulator of cell proliferation, differentiation, and transformation [38]. Depending on the cell type and environment, c-Fos can function either as a transcriptional activator or as a transcriptional repressor. According to Bland et. al [39] increased c-Fos expression in breast cancer was associated with poor prognosis. Gee et. al [40] have observed a significant association between elevated Fos protein expression and increased proliferation. Furthermore, Fra-1, Fos family member and an AP-1 transcription factor component is known to induce promoter activity of several target genes, such as MMP-1 and MMP-9 and involved in cell proliferation and invasiveness of breast cancer cells [41, 42].
Substantial experimental studies indicated that many dietary natural products could affect the development and progression of breast cancer, such as soy, pomegranate, mangosteen, citrus fruits, apple, grape, mango, cruciferous vegetables, ginger, garlic, black cumin, edible macro-fungi, and cereals [43]. In general, fruits and vegetables contain various dietary substances such as vitamin, fiber, phytochemical and phenolic compounds like flavonoids and vanilloids, which act as anti-cancer agents [43]. Their anti-breast cancer effects involve various mechanisms of action, such as downregulating ER-α expression and activity, inhibiting proliferation, migration, metastasis and angiogenesis of breast tumor cells, inducing apoptosis and cell cycle arrest, and sensitizing breast tumor cells to radiotherapy and chemotherapy [43, 44].
Soybean is an excellent source of dietary phenolic substances in the Asian diet [45, 46]. The two major isoflavones in soybean are genistein and daidzein. It has been reported that growth inhibitory effect of genistein may be linked to suppression of angiogenesis [47, 48] and cell cycle progression as well as stimulation of apoptosis [49]. Genistein has been shown to attenuate the transcription of c-Fos mRNA in breast cancer cells [50, 51].
Extracellular matrix metalloproteases such as MMP-1, MMP-3, MMP-7, and MMP-9 and the fibrinolytic system such as uPA (urokinase-type plasminogen activator) are important protease systems interacting with each other in charge of remodeling and recycling of tissues and they play important role in tumor invasion and metastasis [39, 51, 52]. A recent study has confirmed the association between MMPs and tumor growth, invasion, metastasis and prognosis of breast cancer 53. Urokinase plasminogen activator receptor (uPAR), also designated CD87, is a glycoprotein surface receptor specific for urokinase plasminogen activator (uPA). Active uPA is extracellular matrix-degrading protease that promotes tumor progression and metastasis. It has been reported that uPAR in serum and in urine of breast cancer patients was significantly higher than in healthy control [54, 55]. An association between a high expression of MMP-2 and reduced survival in breast cancer patients as well as an association of the tumor grade with increased levels of MMP-9 in breast cancer tissue was reported [56]. However, despite increasing knowledge about the physiological functions of AP-1, how upstream signaling pathways, mainly MAPKs, regulate the transcriptional activity and how the proteins of the Fos and Jun families give rise to AP-1 signaling pathway in DMBA-induced rat breast tumors and how differential effects are achieved between soy protein treated group and casein treated group on MAPK/AP-1 signaling pathway in DMBA-induced rat breast tumors are not fully elucidated. To our knowledge, currently there are no data available on the effect of casein and soy proteins on the level of PCNA, cyclin D1, MMP-9 and uPAR in DMBA-induced rat breast tumors. Therefore, our specific aim was to evaluate the role of MAPK phosphorylation and subsequent AP-1 activation on the differential effects of soy protein and casein on the development of aggressiveness and progression of DMBA-induced breast tumor and whether soy protein control MMP-9 and uPAR expression by modifying AP-1 activity in DMBA-induced breast tumor.
Development of breast tumor in female rats
Adult female Sprague Dawley rats were purchased at 22 days of age from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). They were housed individually in polycarbonate cages. The method was described in detail previously [24]. Briefly, animals were divided into three groups. Each group contained 10 animals. Animals in groups 1 and 2 were fed a standard AIN-76A diet containing 20 percentage casein as protein source and those in group 3 were fed with same diet replacing casein with 20% soy protein as protein source. The diets were prepared by Harlan Teklad (Madison, WI) [23, 24].
The rats were placed on the either casein (group 2) or soy protein (group 3) at 25 days of age and remained on the same diet for the rest of the study. Rats were allowed to feed and drink ad libitum. Mammary tumors were induced in rats of groups 2 and 3 by a single intragastric administration 80 mg/kg b.wt. of dimethylbenz[a]anthracene (DMBA; Sigma-Aldrich Chemical Co, St. Louise) in sesame oil at 50 days of age. Control animals (group 1) received the vehicle only by gavage. This group served as a control for group 2 and 3, which received DMBA. Since our previous study indicated that replacement of casein by soy protein had no effect on either morphology or biochemical characteristic in breast tissue of rats, which did not receive DMBA [23, 24]. Hence, we used casein protein in control group.
Animals were weighed and also palpated triweekly to detect tumors beginning four weeks after the administration of carcinogen. At almost 120 days post-administration of DMBA, animals were sacrificed by CO2 asphyxiation. All tumors were weighed and measured for volume, and a section of the tumor was fixed in buffered formalin pH 7.5. Sections of the paraffin-embedded tumors were stained with hematoxylin (H) and eosin (E) for morphological analysis. The remaining tissues were stored at -80oC for biochemical studies. For biochemical studies, we took tissues from three different tumors (in case of casein group Grade I, II and III) and mixed them and designated them as one sample (n=5)
Histological grading of mammary gland adenocarcinoma
The slides were examined and scored as described earlier [23]. The final grade was determined by the total scored based on the tubule formation within the neoplasm, nuclear pleomorphism and mitotic count per 10 high power field (hpf). The tumor was scored 1-3 depending on the number of mitotic figures per 10hpf. Using these parameters mammary gland adenocarcinomas were graded I, II and III. Grade I was less aggressive and had the best prognosis and grade III was the most aggressive and had the worst prognosis [23].
Immunohistochemical staining of Ki-67 for assessment of mitotic activity
Formalin (10%) fixed sections were incubated with 0.3% H2O2 to block endogenous peroxide activity. The sections were washed and incubated with normal rabbit serum for 30 min to block non-specific binding. Afterwards, the sections were incubated with a polyclonal antibody for Ki-67 (diluted 1/50 in PBS, DAKO). The sections were washed and then incubated with biotinylated secondary antibody for 1 h. The sections were washed and incubated with HRP-Streptavidin for 1 h at room temperature and reacted with diaminobenzamidine until reddish brown color developed. Sections were then counterstained with Gill’s hematoxylin solution, and cover slipped. Ki-67 reactivity was evaluated by counting the number of positive and negative epithelial cell nuclei in six randomly selected fields in each section (n=5) [23].
Assay of MAPK (Total/Phospho) multispecies assay by enzyme-linked immunosorbent assay (ELISA)
The breast tissues were homogenized with cell extraction buffer (Biosource International, Inc., Camarillo, CA) and centrifuged for 10 minutes at 10,000 rpm. Protein concentration of supernatant was measured by Lowry method [57] and stored at -80oC for further analysis. The MAPK activity in the breast tissues was measured by ELISA kits (Biosource International, Inc., Camarillo, CA) following the protocol provided by manufacturer. The absorbency at 450 nm was performed by a Benchmark Plus Microplate Spectrophotometer (Bio-Rad, Hercules, CA).
AP-1 activation assay
The activation of DNA binding activity of individual family member of AP-1 was quantified by ELISA using the AP-1 transcription factor family assay kit (Active Motif, Carlsbad, CA), according to the manufacturer’s protocol. Briefly, the breast tissues were homogenized in a 5 volume of ice-cold tissue lysis buffer (Biosource International, Inc, Camarillo, CA) containing protease inhibitor cocktail (0.01%, Sigma–Aldrich Chemicals, St. Louis, MO) with 0.1 mM PMSF and incubated in 96-well plates to which oligonucleotide containing a TPA responsive element (TRE) was immobilized. AP-1 binding to the target oligonucleotide was detected by incubation with primary antibodies specific for c-Fos, Fos-B, Fra-1, Fra-2, p-c-Jun, JunB, JunD and followed by subsequent incubation with secondary anti-IgG horseradish peroxidase conjugate and visualized with developing solution and quantified at 450 nm with a reference wavelength of 655 nm by a Benchmark Plus Microplate Spectrophotometer (Bio-Rad, Hercules, CA) [58].
Western blot Analysis
Breast tissues were homogenized in a 5 volume of ice cold 210 mM mannitol, 70mM sucrose, 10 mM Tris-HCl, 1mM EGTA, 2mM CaCl2 buffer, pH 7.2 containing protease inhibitor cocktail (0.01%, Sigma–Aldrich Chemicals, St. Louis, MO) by using a Brinkman Polytron (setting 6-7, 30 sec). Proteins were measured by Lowry method [57] and 50mg protein was separated by 12% SDS polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred electrophoretically onto PVDF membranes Immobilon-P (Millipore, Bedford, MA). The membrane was immunoblotted with primary antibodies of c-Fos, FosB, Fra-1, Fra-2, c-Jun, JunB, JunD, ATF-2, L-ZIP, cyclin D1 and proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology Inc., CA) primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibody. Binding of antibodies to the blots was detected with an ECL-detection system (Perkin Elmer, Boston, MA) following manufacturer’s instructions. Stripped blots were re-probed with b-actin specific polyclonal antibodies (Santa Cruz Biotechnology Inc., CA) to enable normalization of signals between samples. Band intensities were analyzed using Bio-Rad Gel Doc (Bio-Rad, Hercules, CA). For MMP-9 and uPA analysis, we used secondary antibody with fluorescent using manufacture’s protocol. Band detection and intensities were analyzed using an Odyssey (LI-COR Biosciences, NE) infrared fluorescent at 680 and 780nm [59].
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from 100 mg of breast tissue using a Qiagen RNAEASY kit (Qiagen, CA) according to manufacture protocol. The concentration and purity of RNA were analyzed using a UV spectrophotometer [59].
RT-PCR of c-Fos and c-Jun and GAPDH was performed using 5µg of total RNA from control, casein and soy sample, using one-step RT-PCR kit (Invitrogen, MD). The primer sequences were: 5’-GCCCAGTGAGGAATATCTGGA-3’ (forward) and 5-ATCGCAGAT GAAGCTCTGGT-3’ (reverse) for c-Fos; 5”-TCGCAGATGAAGCTCTGGT-3’ (forward) and 5’-GGCCATCTCTTGCTCGAAGTC-3’ (reverse) for c-Jun and 5’-GGTATCGTGGAAGGACTCATGACC–3 (Forward) and 5’-TCGCTGTTGAAGTCACAGGACACA –3’ (Reverse) for GAPDH). RT-PCR was performed in a thermal cycler (Biometra, TRIO gradient) as follows: 1 cycle for 30 min at 450C, 1 cycle at 940C for 2 min, 39 cycles for 1 min at 940C, 1 min at 500C, 2 min at 720C and 1 cycle for 10 min at 720C. The RT-PCR products were electrophoresed on a 1% agarose gel and band intensities were quantified using Bio-Rad Gel Doc (Bio-Rad, Hercules, CA). Then bands are purified using QIA quick PCR purification kit (Qiagen, CA).
Statistical analysis
Data were expressed as means ± standard error of mean (S.E.M.). Statistical significance was determined by the student’s t-test or Dunnett’s test after one-way analysis of variance (ANOVA), using GraphPad prism Version 9.0 (GraphPad software, SanDiego, CA). Results were considered statistically significant at p is less than 0.05.
Representative photograph of light microscopy from breast tissue of control animals regardless of whether they were fed casein protein is shown in Figure 1A-1C. It reveals scattered normal lobuloalveolar units lined by a layer of cuboidal epithelium and a discontinuous layer of flattened myoepithelial cells and terminal end buds in an adipose tissue stroma (H & E x 40). No tumor was noticed in any control animal. Figure 1B shows grade III modified Scarff-Bloom-Richardson (SBR) mammary duct carcinoma in casein group. This tumor is composed of sheets of infiltrating tumor without tubular elements comprising more than 75% of the tumor cells arranged in solid structures. The nuclei are crowded, enlarged, pleomorphic, with dense finely to coarsely granular chromatin, and visible nucleoli. More than 20 mitoses per 10 hpf at x 40 are present in this animal. Areas of tumor necrosis with polymorphonuclear neutrophil, lymphocyte, and plasma cell infiltrates are present.
Representative photograph of light microscopy from breast tumors found in the DMBA/soy protein group are shown in Figure 1C (H & E x 40). It reveals the tumors to be composed of cells arranged in isolated small round to oval elongated tubules lined by columnar epithelial cells with one or two layers of basal nuclei. The lining epithelial cells exhibit enlarged, crowded, uniform, hyperchromatic nuclei with evenly disbursed finely granular chromatin and indistinct nucleoli. The basal nuclei of the tubular epithelium show an absence of myoepithelial cells, indicating infiltrating neoplastic cells. The tubules vary in size and shape, are mostly isolated, widely spaced, and surrounded by a copious amount of fibrous connective tissue. Occasional tubules show little intervening stroma. The solid areas displayed in the lesion are less than 25% of the lesion. This lesion is comparable to a low-grade tumor, grade I modified Scarff-Bloom-Richardson mammary duct carcinoma.
The miotic activity assessed in grading the tumors was verified by immunohistochemical reactions for Ki-67 antibody (Figure 1D -1F). Tumors from animals in the DMBA/casein group (Figure 1E) had more 3.0-fold of the cells staining positive with Ki-67 antibody than control (Figure 1D). However, tumors from the animals of the DMBA/soy protein group showed 1.4-fold increase of the cells staining with Ki-67 antibody (Figure 1F) than control group. Thus, proliferative index was significantly lower (2.8 fold) in the soy protein group than that in the casein group (Figure 1G).

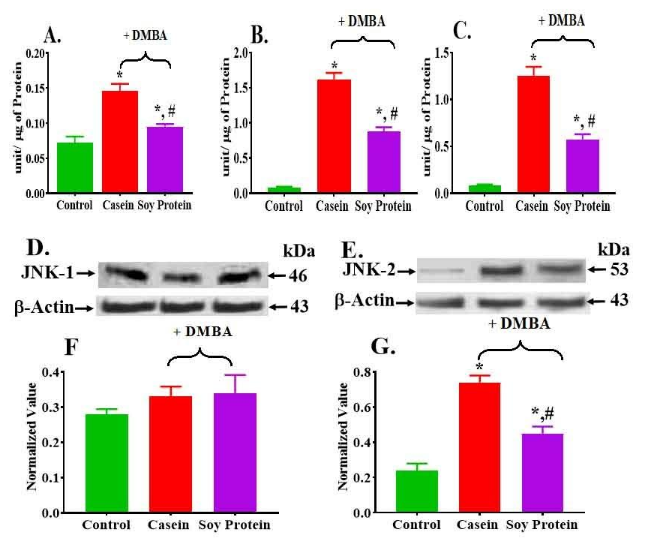
Tumor had higher activity of ERK1/2, p38 and JNK than control (Figure 2A-2C). However, tumors in the animals fed soy protein had significantly lower total levels and activities for all MAPKs than animals fed casein (Figure 2A-2C, Table 1).
| Tumor Group | Normalized with respect to | |||
| ERK 1/2 | P38 | |||
Ratio (Phospo/Total) | % Change | Ratio (Phospo/Total) | % Change | |
| Casein | 0.72 | 52.78↓ | 7.53 | 34.5↓ |
| Soy protein | 0.34 | 4.93 | ||
Table 1: Effects of casein and soy protein on mitogenic activated kinase (MAPK) activity in Brest with or without DMBA treatment
The activity of ERK1/2 increased 1.4-fold in casein than control, whereas it decreased 1.7-fold in soy group than casein (Figure 2A). Furthermore, the ratio of PhosphoERK1/2 vs total decreased 2.1-fold in tumor from soy group than casein group (Table 1). Figure 2B shows the total of p38 MAPK activity. Both total p38MAPK activity and ratio of phospho p38MAPK vs total p38 MAPK increased 1.9-fold and 7.5-fold in casein group than control, whereas it decreased 1.8-fold and 1.5-fold in soy group than casein respectively (Figure 2B, Table 1). The total JNK1/2 activity increased 14.7-fold and 6.7-fold in both casein and soy group than control (Figure 2C, Table 1) whereas both total and ratio of phospho JNK1/2 decreased in soy protein group 2.2-fold and 1.6-fold than casein respectively (Figure 2C, Table 1). Tumors of both casein and soy groups had higher phospho ERK 1/2, phospho p38 and phospho JNK1/2 activity than corresponding control (Table 1. However, tumors in animals fed soy protein had significantly lower level of activities for all phospho MAPKs than animals fed casein (Table 1). Since we observed a significant increase in the activity of the JNK family in tumors, we measured the protein level of individual members of the JNK family (JNK-1 (D, F) and JNK-2 (E, G) in Figure 2). While no significant change in the protein level of JNK-1 (Figure 2F) was observed, a significant increase in JNK-2 (Figure 2G) was observed in the tumors of animals fed either casein (3.1 fold) or soy protein (1.9 fold) than control (Figure 2G). But this level was much elevated in the casein group than soy protein group (1.7 fold decreased than casein, Figure 2D -2G).
According to Figure 3A, DNA binding activities of members of Fos family, such as c-Fos (1.5 fold), FosB (1.3fold), Fra-1 (1.4fold), Fra-2 (1.1 fold) and members of Jun family such as Jun B (1.2 fold) and (JunD, 1.7fold)) was significantly higher in tumors of animals fed casein than corresponding control (Figure 3A). However, this increased activation of c-Fos (1.2 fold), FosB (1.3 fold), Fra-1 (1.3 fold) and JunD (1.2 fold) in tumors was downregulated when casein was replaced by soy protein in diets, but c-Fos and JunD activity increased in tumor of soy group from the control (Figure 3A).
Since we observed higher activation of AP-1 transcription factor in casein group than soy protein group, we determined the protein levels of individual members of AP-1 family. We found a significant increase in ATF-2 (2.9 fold and 2.0-fold; Figure 3B, 3D) and L-ZIP (5.9 fold and 2.4-fold; Figure 3C, 3E) protein expression in the tumors of animals fed either casein or soy protein respectively. But this level was much elevated in tumors of casein group (1.4 fold and 2.5-fold) than soy protein group (Figure 3B-E).

We also observed a significant increase in the protein levels of c-Fos (1.8 fold; Figure 4A, 4D), Fos-B (1.8 fold; Figure 4B, 4E) and Fra-1(2.0 fold; Figure 4C, 4F) in tumors of casein group than control breast tissues. But this level was significantly decreased (1.8-fold, 1.7-fold and 2-fold respectively) when casein is replaced by soy protein (Figure 4A- 4F). No significant change was observed in Fra-2 protein expression between tumors and control breast tissues (data not shown). A significant increase in the protein levels of c-Jun (2.3 fold and 2.9-fold; Figure. 4G, 4J), Jun-B (4.9 fold and 4.4-fold; Figure. 4H, 4K) and Jun-D (5.9 fold and 4.9-fold; Figure 4I, 4L) was observed in both casein and soy group tumors respectively than control breast tissues (Figure 4G -4L). However, no significant difference was observed between casein group and soy protein group (Figure 4G -4L).
Since we observed significant variation of c-Fos and c-Jun level in tumor of both casein and soy protein groups, we measured the expression of both c-Fos and c-Jun in both tumors and control groups. The band densities obtained for c-Fos and c-Jun after normalization with that of GAPDH are shown in Figure 4M to 4P. We observed a significant increase (1.6 fold) increase in c-Fos gene expression in tumor of casein group than control (Figure 4M, 4N). c-Fos expression was decreased 1.5-fold in tumor when casein is replaced by soy protein (Figure 4M, 4O), whereas c-Jun expression was significantly higher in both casein (1.5 fold) and soy protein group (1.8 fold) respectively than control (Figure 4N, 4P).

Emerging evidence suggests that PCNA is at the very heart of many essential cellular processes, such as DNA replication, repair of DNA damage, chromatin structure maintenance, chromosome segregation and cell-cycle progression. Cyclin D1 is an important regulator of the cell cycle progression and development of cancer. To further explore the involvement of AP-1 in cell proliferation and cell-cycle progression, we determined the protein levels of cell cycle proteins, PCNA (Figure 5A, 5C) and cyclin D1 (Figure 5B and 5D). The data obtained after normalization with β-actin revel that the protein levels of both PCNA (2.0 fold) and cyclin D1 (7.0 fold) were significantly higher in casein tumors than control breast tissues (Figure 5A-5D). However, the levels PCNA (1.2 fold) and cyclin D1 (1.4 fold) were significantly lower in soy protein group than casein group (Figure 5A-5D).

Matrix metalloproteinases (MMPs) have been implicated in diverse roles in breast cancer development and progression. While many of the different MMPs expressed in breast cancer are produced by stromal cells MMP-9 is produced and AP-1 was the crucial transcriptional factor for expression of MMP-9 and uPA. Hence, we explore whether there is any differential effect between casein and soy protein on the level of MMP-9 (Figure 5E, 5G) and uPA (Figure 5F, 5H) in DMBA-induced breast tumor. Figure 5E-5H show that the level of MMP-9 and uPA were increased 2.7-fold and 4.2-fold respectively at casein group than control, but it decreased 2.0-fold and 2.4-fold respectively, wen casein is replaced by soy protein (Figure 5E-5H).
Previously, we reported that total replacement of casein protein with soy protein causes not only causes delay initiation and progression but also decreased the number of breast tumor present in the rat [23]. To further explore whether the combination of casein and soy protein has any effect on tumor incidence and total number of tumors in DMBA-induced rat breast. Our preliminary data indicated that 10% casein with 10% soy also causes gradual delay the time course of palpatable tumor incidence (Figure 6A) than 20 percentage casein only. Both number of rats with tumor (Figure 6B) and the number of tumors per rat (Figure 6C) decreased as soy protein replace casein. The initiation of tumor development was observed as follow 20 percentage Casein (Group 1) <10>

Breast cancer is the most common cancer among women in both developed and less-developed countries. According to GLOBOCAN, cancer is a major health problem worldwide. The number of cases increases every year and rates in countries with high consumption of soybeans and whey are lower than those in the United States. Barak and Fridman [60] reported that the people who adhere to the Mediterranean Diet have lower incident of cancer. We previously reported that both soy protein and α-lactalbumin, which is one of the active components of whey, has a beneficial effect on the initiation, progression and development of more aggressive breast cancer [23, 61]. The aim of this study was to not only validate our previous observation, but also elucidate the possible mechanism by which soy protein mediates its delay the initiation tumor and protective effect on the development of aggressive breast tumors. We observed that 100% of the tumors in DMBA/soy protein group was non-aggressive (Grade I) where as DMBA-casein group higher percentage of aggressive tumors (Grade I > Grade II > Grade III) of aggressive tumors as reported previously by us 23. Based on recent a large cohort data analysis of a clinical cancer registry, it was demonstrated that Ki-67 is frequently determined in clinical study for the prediction of the risk of reoccurrence 62. We observed that the number of Ki-67 positive staining intensities i.e., the proliferative activity of the tumor per field significantly increases in DMBA/casein group where as it was significantly reduced in DMBA-soy group than that in casein group (Figure 1G). Positive Ki-67 staining correlates with degree of differentiation, vascular invasion, lymph node metastasis and aggressiveness of the tumors as reported by us and others [23, 61-63]. Recent studies also indicated that the proliferation biomarker Ki-67 is also considered as a prognostic factor for breast cancer and level can be used as a valuable biomarker in breast cancer patients [64, 65]. Our present study also demonstrate that there was a strong correlation between grading (Figure 1A-1C) and Ki-67 (Figure 1D-1G) and validate our previous observation that the DMBA-soy protein group contained lower grade tumor than DMBA-casein group. This result indicates that when casein protein was replaced with soy protein not only significantly and physiologically delay development of mammary tumor in rats exposed to DMBA but also inhibits the aggressiveness of tumor progression.
To identify the signaling pathways that is involved in mediating this differential effect of casein and soy protein on mammary gland, we measured the signaling cascade of individual members of both total and phosphoMAPK family i.e., ERK (Figure 2A), p38 (Figure 2B), and JNK (Figure 2C, Table 1) in the breast tissues. According to our results, a significant increase was observed both the activity of total and phosphoMAPKs (ERK1/2, p38 and JNK1/2) was higher in tumor of both DMBA-casein and DMBA-soy than control breast tissues. Soy protein showed a significant decrease in the activity of all MAPKs (Figure 2, Table 1). Western blot analysis revealed that it was JNK-1 (Figure2D, 2F) and not JNK-2 (Figure 2E, 2G) was increased in both DMBA-Casein and DMBA-soy protein group tumors than control breast tissues (Figure 2D-2G), but soy protein decreased significantly the protein level of JNK-2 in comparison to the DMBA-casein group (Figure 2E, 2G). Chen et. al [66] reported that ERK plays a significant role in progression of tumor. Recent report indicates that rapidly accelerated fibrosarcoma/mitogen-activated protein kinase (MEK)/ERK signaling pathway is significantly correlated with the clinicopathological features and prognosis for patient’s breast cancer having axillary lymph node metastasis [67]. Studies have shown that p38MAPK promotes breast carcinoma invasion and metastasis [67]. Multivariate analysis indicates that triple negative breast cancer (TNBC) patient with ERK-2 – overexpressing tumor had a lower overall survival rate than those with low ERK-2-expressing tumors. ERK-2 and pMAPK are valuable prognostic markers in TNBC. Further studies are justified to elucidate ERK’s role in TNBC tumorigenicity and metastasis [68]. Current evidence clearly shows that MAPK pathways such as JNK and p38 play important role in the not only enhance the efficacy but also development resistance and toxicity of chemotherapeutic drugs [69]. P38MAPK signaling in tumor cells promotes breast carcinoma growth and metastasis by altering the tumor microenvironment (TME). Inactivation of p38MAPK signaling in breast carcinoma cells reduced growth and spontaneous metastasis of tumor xenografts [70]. The present study indicates that soy protein significantly inhibits all three MAPKs activity and can provide new therapeutic options for treatment of breast cancer including metastasis disease.
Distinct MAPK pathways are responsible for the phosphorylation and activation of AP-1 proteins [71]. The stress-responsive p38 and JNK MAPK pathways regulate cell cycle and apoptosis [72]. MAPKs have significant roles in mediating signals triggered by cytokines, growth factors, and environmental stress; and are involved in controlling cell proliferation, cell differentiation, and death [73, 74]. Recently, it has been reported that soy sauce induced mitochromesis and increased oxidative stress tolerance, which is mediated through p38 MAPK pathway [75]. Development and progression of breast tumors involve a complex series of events, including oxidative stress, dysregulation of cellular differentiation, excessive proliferation and resistance to apoptosis [76]. Moreover, our laboratory reported that antioxidant liposome significantly counteracted the half mustard gas-induced oxidative stress and activation of AP-1 transcription factors by modulating Fos, Jun and ATF family as a result it protects form mustard gas induced lung injury [58]. Oxidative stress is a crucial factor for cancer progression and therapy. Several studies have shown that DMBA can be used to induce experimental breast carcinomas in rats and that this process involves disruption of tissue redox balance; in turn, this suggests that biochemical and pathophysiological disturbances may result from oxidative damage [77, 78]. Soy, phytochemicals and other antioxidant intake decreases oxidative stress as well as their ability to induce apoptosis in cancer cells [79, 80]. Our result suggests that soy protein may have imparted its beneficial effects by controlling the MAPK pathway. The precise nature of the effect of oxidative stress on cancer development and/or response to treatment with soy protein requires further exploration.
AP-1 is the downstream signal of MAPKs signaling pathways and the blocking of MAPKs leads to the inhibition of AP-1 transactivation and subsequent cell transformation [34]. AP-1 activity plays a critical role in the process of tumorigenesis [35]. AP-1 transcription factors are targeted by many signal transduction pathways and regulate a multiple of cellular processes, such as cell proliferation, survival, differentiation, invasion and carcinogenesis, depending on their dimer composition [80-83]. To elucidate the downstream signal of MAPK pathway, we monitored the protein levels of AP-1 family. Since AP-1 is a homo or hetero dimers of Fos family, Fos/activating transcription factor (ATF) and Jun subfamilies of basic-region leucine-zipper (L-ZIP) proteins, we measured the protein levels of individual components. For ATF, we studied only ATF-2 since it is the predominant form in vertebrate. The protein levels of Fos/activating transcription factor (ATF-2) and Jun subfamilies of basic-region leucine-zipper (L-ZIP) proteins were higher in tumor samples from casein group than corresponding controls. The protein level of both ATF-2 (Figure 3B, 3D) and L-ZIP (Figure 3C, 3E) were decreased if casein was replaced by soy protein. It has been reported that pATF2 has been shown to facilitate the transcription of MMP2, which increases migration in H-Ras-transformed MCF10A human breast epithelial cells indicating that ATF2 may play a role in breast cancer metastasis [84]. It has been also reported that Knocking ZIP expression down in breast cancer cells leads to dysregulated proliferation, indicating its potential role as a tumor suppressor [85]. However, whether ATF-2 and ZIP play important role in delay initiation and progression of breast tumor by soy protein requires further exploration.
We also measured the protein level of individual family members of both Fos (c-Fos, Fra-1, Fra-2 and Fos B) and Jun (c-Jun, Jun B and Jun D). The protein levels of certain family members were higher in tumor samples than corresponding control (Figure 4). Soy protein caused a decrease in the protein levels of c-Fos, Fra-1 and Fos-B (Figure 4A-4F) and also members of Jun family Jun B and Jun D (Figures 4H, 4K and 4I, 4L) except Jun C (Figure 4G, 4J). It has been reported that there was significant association between increased Jun B expression and reduced tumor size and tumor stage [82]. Jun and Fos proteins differ significantly in both their DNA binding and transactivation potential as well as their target gene regulation [86]. In vitro studies have shown that Jun/Fos heterodimers are more stable and have a stronger DNA binding activity than Jun homodimers [87, 88]. Although soy protein increased the expression of c-Jun (Figure 4N, 4P) but the c-Fos expression in the tumors of the soy protein group was at the basal level (Figure 4M.4O). Since c-Fos and c-Jun dimerization is necessary to activate AP-1, we conclude that activation of AP-1 is higher in tumors from casein group than that in tumors from soy protein group. There is noutationn in c-Fos or c-Jun gene in tumor of both casein and soy group than normal. Sundqvist et al [89] reported that the AP-1 component Jun B was requires for expression of many late invasion mediating genes, creating a feed-forward regulatory network that aggravates breast cancer invasion. Recent report also shown that the AP-1 member Fra-1 expression level is correlated with the expression of genes that have been implicated in cancer, influences cell proliferation, in vitro cell invasiveness and motility of breast cancer cells as a result it play a pivotal role in breast cancer progression and aggressiveness of TNBC [90, 91]. The present study indicates that replacement of casein protein by soy protein significantly decreased c-Fos, Fos B and Fra-1 (Figure 4A-4F) level in DMBA-induced rat breast tumor. Hence, this is the first report that consumption of soy protein rather than animal protein modulates both AP-I and MAPK signaling and as a result it delays the initiation and aggressiveness DMBA-induced rat breast tumor.
Proliferating cell nuclear antigen (PCNA) is a cofactor of DNA polymerase delta and is required for DNA synthesis. The PCNA gene contains AP-1 sites in the promoter region and its expression is regulated by AP-1 activity [91]. Cyclin D1, the regulatory subunit of several cyclin-dependent kinases, is required for, and capable of shortening, the G1 phase of the cell cycle. AP-1 proteins bind the cyclin D1-954 region. Cyclin D1 promoter activity was stimulated by over expression of mitogen-activated protein kinase through the proximal 22 base pairs [92]. Several AP-1 proteins are shown to bind these sites and activate cyclin D1 expression [93]. Our result shows an up regulation of both PCNA and cyclin D1 in DMBA- exposed animal fed casein compared with control (Figure 5A -5D). But these increase of PCNA and Cyclin D1 are down regulated when casein is replaced by soy protein (Figure 5A-5D). Hence, our results show that replacing casein with soy protein down regulate AP-1 dependent cell cycle proteins as well as cell differentiation marker animal exposed to DMBA (Figure 5A-5D) as a result it inhibits tumor cells proliferation and differentiation.
The progression, invasion and metastasis are augmented by proteolytic enzymes like metalloproteinase and serine protease, because they degrade the basement membrane enabling the tumor cells to invade the adjacent tissues [94]. In the present study we observed that casein significantly increased both MMP-9 and uPA level (Figure 5E-5H) than control, whereas replacing casein protein with soy protein significantly decreased the level of MMP-9 and uPA in DMBA-induced rat breast tumor. Recent meta-analysis demonstrated that overexpression of both MMP-2 and MMP-9 in tumor was associated with larger tumor size, lymph node metastasis and poor prognosis [54, 57]. It has been reported that the uPA activates many intracellular signaling and play important role in inflammation and tissue remodeling [95] and overexpression of uPA enhanced malignant potential in TNBC [96]. Moreover, simultaneous knockdown of both uPA and MMP-9 not only reduced breast cancer progression but also decreased the epithelial cells to mesenchymal transition (ENT) in the tumor micro-environment by altering the expression of ENT genes [97]. In the present study, we observed that soy protein significantly decreased the level of both MMP-9 and uPA in DMBA-induced rat tumor (Figure 5E-5H) and aggressiveness of tumor (Figure 1). However, the precise mechanism of action of soy protein in modulation of uPA and MMP-9 function on regulation of aggressiveness breast cancer and whether deactivation of MAPK pathway and /or down-regulation of AP-1 by soy protein requires further investigation.
We previously reported that replacing casein with soy protein not only delay tumor initiation and decreased tumor size and number, but also inhibited progression of higher clinical stage of tumors. In the present study, we observed that feeding 10% casein plus10% soy protein delay tumor initiation in rat after DMBA post-treatment (Figure 6 A) than 20 percentage casein group but earlier than 20% soy protein group. Furthermore, we also observed that both numbers of rat with tumor and number of tumor present in each rat varies as follow 20% casein (group 1) > 10 percentage casein plus 10% soy protein (group 2) > 20% soy (group 3) (Figure 6B and 6C). Furthermore, we also observed that both tumor size and clinical grading of rat tumor decreased with gradual replacing casein with soy protein as follow 20 percentage casein (group 1) less than 10 casein plus 10% soy less than 20 percentage soy only (group 3) respectively (data not shown). Hence, our preliminary data indicates that total replacement of casein by soy protein is required for delay initiation, progression and aggressiveness of DMBA-induced rat breast tumor, but needs further validation.
The present study demonstrates that the beneficial effect of soy protein in breast cancer prevention may be mediated by control of MAPK/AP-1 pathway. We conclude that deactivation of MAPK pathway lead to down-regulation of the AP-1 activation which in turn down regulates the target gene and may responsible for the beneficial effects of soy protein. Possible mechanism of action by which soy protein modulate MAPK/AP-1 signaling in DMBA-induced breast cancer are shown in Figure 7.

Furthermore, our findings suggest that 38MAPK, MMP-9 and uPA may be a potential target for anticancer therapy inhibiting tumor vasculature and invasion stimulated by tumor-associated stroma.
Soy protein diets have been associated with lower risk of various diseases, including type 2 diabetes, cardiovascular disease, and other cardiometabolic risk factors. However, the association between soy protein diet quality and breast cancer remains unclear. This study provides evidence that adherence to a healthful soy protein diet may reduce the risk of breast cancer, especially those that are more likely to be aggressive tumors. Moreover, total replacement of animal protein by soy/plant protein may provide a new therapeutic option for treatment/management of breast cancer, including metastatic disease.
Funding Information
This study was supported by the US Army Grant DAMD 17-03-1-0352, FAMRI grant 02416, NIH grant 5U54MD007953 and RCMI5U54MD007586-34.
Authors’ contributions
Salil K. Das and Shyamali Mukherjee designed the experiments. Sutapa Mukhopadhyay and Shyamali Mukherjee conducted the experiments. Shyamali Mukherjee and Salil K. Das wrote the manuscript, and all authors participated in the review and approval of the final manuscript.
Conflict of interests
The authors declare no conflicts of interest.
Data availability statement
All data generated or analyzed during the present study are included in these articles.
ORCID
Salil K. Das https://orcid.org/0000-0002-4907-5252
Shyamali Mukherjee https://orcid.org/0000-0003-3357-7079
Ethics approval and consent to participate
Ethical approval was obtained from the Institutional Review Board (IRB) and Institutional Animal Care and Use Committee (IACUC) prior to commencing this study.
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.
Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
