AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2641-0419/164Copyright
*Corresponding Author: Ujjwal Kumar Chowdhury, Professor Department of Cardiothoracic and Vascular Surgery AIIMS, New Delhi-110029, INDIA.
Citation: Ujjwal Kumar Chowdhury, Niwin George, Lakshmi Kumari Sankhyan, Suryalok Angadi, Suruchi Hasija, et al (2021) Tracheostomy in Infants after Cardiac Surgery: Indications, Timing and Outcomes. J. Clinical Cardiology and Cardiovascular Interventions, 4(10); Doi:10.31579/2641-0419/164
Copyright: © 2021 Ujjwal Kumar Chowdhury, This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 01 April 2021 | Accepted: 12 May 2021 | Published: 18 May 2021
Keywords: congenital heart disease; diaphragmatic paralysis; mechanical ventilation; outcome assessment; trachcheostomy; tracheobronchomalacia
Objective: There is little consensus on the indications and optimal timing of tracheostomy in the pediatric population. Our primary aim was to determine if early tracheostomy improves patient outcomes (between 10th and 15th postoperative day).
Methods: A retrospective review of 84 neonates and infants requiring tracheostomy after cardiac surgery between January 1997 and December 2019 was performed. Indications and timings for tracheostomy, and risk factors for mortality were analyzed using Cox regression analysis. The receiver operating characteristic curve analysis, Youden’s index, sensitivity and specificity plot were performed to determine the optimal cut-off point of the timing of tracheostomy.
Results: Twenty-five (29.76%) neonates and 59 (70.23%) infants with a median weight 7.6 kg (IQR: 3.1-9.25 kg) were studied. Extubation failure and unsuccessful weaning from ventilator occurred in 45 (53.6%) and 39 (46.4%) patients respectively. The timing of tracheostomy of 15 days as the optimal cut-off point was associated with a sensitivity of 73% and a specificity of 84% and a Youden’s index of 0.60. Early tracheostomy was associated with decreased mortality (p<0.001), morbidity (p<0.001), decreased duration of ventilation (p<0.001), ICU length of stay (p<0.001) and decreased time of decannulation (p<0.001).
The hazard of death was 5.26 times (95% CI: 1.47-20.36) higher in patients undergoing late tracheostomy. At a median follow-up of 166 (IQR: 82.5-216) months, the actuarial survival was 86.61%±0.04%.
Conclusions: Early tracheostomy within 15th postoperative day was associated with lower perioperative and late mortality, morbidity and ICU stay compared with tracheostomy between 15-30 days, and confers significant long-term advantages.
Running head: Tracheostomy in infants
Early tracheostomy between 10th and 15th postoperative day in infants is associated with lower perioperative and late mortality and improves patient outcomes.
Perspective Statement
Early tracheostomy between 10th and 15th postoperative day in infants avoids mediastinitis, minimizes subglottic stenosis, and is associated with lower mortality compared with late tracheostomy.
Legend: Results of tracheostomy in infant after cardiac surgery
Abbreviations and Acronyms
Chd :Congenital heart diseases
ECmO :Extracorporeal membrane oxygenation
ICU :Intensive care unit
IQR :Interquartile range
LVEF :Left ventricular ejection fraction
Tracheostomy is performed in 1.3% to 2.7% of pediatric patients following cardiac surgery due mainly to requirement for prolonged mechanical ventilation and various other reasons. [1-9]Children requiring tracheostomy have a higher risk of adverse events and mortality secondary to their comorbidities. [1-9]
Tracheostomy in pediatric patients has been shown to reduce total mechanical ventilation time, shorten intensive care unit and hospital stays, decrease the occurrence of lower respiratory tract infection (LRTI), improve oral and dental hygiene, reduce in-hospital mortality and hospital cost. [1-16] In addition, it decreases the number of self extubations and extubation-reintubation cycles, requirement of sedation, upper airway injury including vocal cord ulceration, dead space ventilation, airway resistance, and work of breathing, therefore facilitating separation from ventilator support in a steady manner. [11-19]
Literature is divided on the definition of early and late tracheostomy. Although tracheostomy is conventionally considered in patients who are ventilator dependent 2 weeks after cardiac surgery, the definition of early tracheostomy in published investigations ranged between 48 hours to 63 days. [1-11,19]
In this study, postoperative day 15 were selected as the upper limit of early tracheostomy and day 30 as the lower limit for the late group, because the likelihood of successful extubation after 30 days of translaryngeal intubation in pediatric cardiac surgery is low and risks of tracheal airway complications increases thereafter. [2,8,12-19]
All patients undergoing tracheostomy between 10th and 15th postoperative days have been grouped under early tracheostomy, and all undergoing tracheostomy between 15th and 30th postoperative days have been grouped under late tracheostomy. In pediatrics, available data on pediatric tracheostomy are limited, and it is difficult to extrapolate data from other centers which practice prolonged mechanical ventilation. [1-19]
Although our institution is a tertiary level center, the socio-economic profile of the patients, and the lack of health insurance benefits in the developing world mandates reduction of duration of postoperative ventilation, and ICU stay, thereby attempting to reduce the cost of treatment. Tracheostomy if performed too early may be associated with increased risk of mediastinitis and if it is delayed, the number of extubation-reintubation cycle, risk of LRTI, and subglottic stenosis is increased. [11-19]
The primary objective of this study was to compare outcomes between early (within 15 days of primary operation) and late (between 15-30 days) tracheostomy. The secondary objectives were to: i) review the indications for tracheostomy in infants undergoing congenital heart surgeries requiring prolonged mechanical ventilation, and ii) evaluate the short- and long-term outcomes in terms of mortality and morbidity in these patients.
This retrospective study conforms to the principles outlined in the declaration of Helsinki and was approved by the Institutional Ethics Committee. Patients were enrolled in the study protocol after obtaining informed written consent from parents/guardians.
A 23-years (January 1997 to December 2019) retrospective review of medical records of all infants who underwent different types of closed and open heart surgical procedures (primary operation) at AIIMS, New Delhi, by the corresponding author, requiring prolonged postoperative ventilation (n=3900) were studied, and patients who underwent tracheostomy (n=84) postoperatively were identified and selected for further review. The six-monthly outpatient medical records were examined for follow-up data (Figure 1).
Criterions for selection of patients, indications and timings for tracheostomy
Decisions for extubation, reintubation, and tracheostomy and decannulation were indeed made collectively by a panel of reviewers comprising of the cardiac intensivist, cardiac surgeon, respiratory therapist and otolaryngologist and remained uniform through the study period. The timing of tracheostomy was tightly linked to patient condition which in turn was linked to postoperative outcome.
Inclusion criteria: In this study on postoperative cardiac surgical patients, the decision/indications of tracheostomy were: i) those requiring prolonged mechanical ventilation following cardiac surgery due to multifactorial reasons, ii) those following failed extubation, and iii) those following second failed extubation. A combination of three of more concerning issues among postcardiac surgical patients requiring prolonged mechanical ventilation was present in more than half of patients in this study (Definitions, Tables 1-3).
Exclusion criteria: Infants with tracheostomy preoperatively, and those requiring prolonged mechanical ventilation but not tracheostomized were excluded. Due to variable surgical and postcardiac surgical practices, patients operated by other surgeons were excluded.
Tracheostomy was performed in the operating room using the open technique in all patients. Postoperative day 15 was selected as the upper limit for early tracheostomy based on the evidences available in the literature, and an increased incidence of postoperative sepsis in the developing world, and to decrease the hospital cost. [1-11] Day 30 was selected as the lower limit for the late group in this study.
Data collection
In addition to baseline characteristics, intraoperative and postoperative findings (table 1), the following mechanical ventilation-related parameters were recorded: i) duration of respiratory support before surgery, ii) the duration of ventilation before tracheostomy, iii) the duration of ventilation after tracheostomy, and iv) time interval between tracheostomy and decannulation or death. The number of attempts at extubation, timing of decannulation (failed or successful), recannulation of tracheostomy, patient’s outcome (decannulation/death) were noted for each patient. The indication(s) for tracheostomy, complications of tracheostomy, decannulation, and their outcomes were also recorded. [20]
Patients were classified with an airway anomaly if they had any major upper airway or laryngotracheobronchial abnormality confirmed by an otolaryngologist, using bedside bronchoscopy and laryngoscopy when clinically suspected. All tracheostomy were done surgically in operation room. Snuggly fitting tracheostomy tube with inflatable cuff was preferred on insertion and later changed to uncuffed tube after one week.
Decannulation was considered when patients had: i) stable hemodynamics with reduced/nil requirement of inotropes; ii) no requirement of ventilator support for a period of 7 days; iii) minimal requirement for suctioning; iv) no pulmonary atelectasis/lobar collapse vi) no diaphragmatic paresis/paralysis; vi) normal kidney function; vii) patent upper airway, ascertained with laryngoscopy vii) removal of any obstructing suprastomal granulation tissue; and viii) no comorbidities necessitating tracheostomy.
Definitions
Primary operation: In this study, we have used the term “primary” operation or procedure to refer to cardiac operation necessitating the period of postoperative ventilation leading to tracheostomy.
Outcome measures: Operative mortality (all cause) was defined as death either in ICU or prior to hospital discharge or within 30 days of the date of surgery according to the congenital heart surgery outcome measures endorsed by the Society of Thoracic Surgeons. [20] Short-term survival was defined as survival to hospital discharge. Long-term survival was defined as survival to most recent follow-up. Intubation time was defined as time in hours from the time of intubation either in the preoperative period or on the day of surgery to discontinuation of mechanical ventilation. Records were reviewed to determine whether patients had been successfully decannulated and if so, to document the time from the date of tracheostomy to decannulation, the length of stay in intensive care unit after surgery, and the need for surgical reintervention, when required.
Extubation failure: Extubation failure was defined as reintubation within 48 hours after extubation. For patients who failed extubation, reintubation was determined according to the following criteria: (i) hemodynamic instability; (ii) respiratory dysfunction (significant hypoxia, worsening hypercarbia, significant respiratory effort, respiratory fatigue, and massive atelectasis); (iii) airway obstruction; (iv) deteriorating level of consciousness; (v) major bleeding; and (vi) cardiac arrest. The decisions for extubation and reintubation were made by attending intensivist, cardiac surgeon and cardiac anaesthetist.
Suboptimal hemodynamics/low cardiac output syndrome: Suboptimal hemodynamic state/low cardiac output syndrome was defined as moderate or severe ventricular dysfunction requiring inotropic support (dopamine at 4-10 µg/[kg/min]), dobutamine at 5-10 µg/[kg/min], epinephrine at 0.01-0.1 µg/[kg/min], milrinone at 50 µg/kg intravenous bolus followed by 0.375-0.75 µg/[kg/min]), either isolated or in combination with or without mechanical circulatory assistance in the operating room or in the intensive care unit, to maintain stable hemodynamics in the absence of residual structural lesions and mechanical external compression after correction of all electrolytes or blood gas abnormalities and after adjustment of the preload to its optimal value. Low-output syndrome was also diagnosed if there was an increasing requirement of the previously mentioned inotropes along with afterload reduction with sodium nitroprusside.
Tracheostomy-related complications: Early complications were defined as those occurring within the first postoperative week. At one week, the tracheostomy has usually matured, and the first tube change is performed. Late complications were defined as those occurring after the first 1st week.
Morbidities: Cardiac morbidity was defined as low cardiac output state with or without the requirement for extracorporeal membrane oxygenation. Renal morbidity was defined as oliguria (<0.5 ml/kg/hr) or anuria with rising blood urea and serum creatinine and/or starting hemodialysis or peritoneal dialysis. Culture proven pneumonia, mediastinitis, wound infection and bacteremia were diagnosed by clinical findings and microbiological cultures, and lab results.
Statistical Analysis
Statistical analysis was performed using STATA 14.0 Software (College Station, Texas, USA). Continuous and interval-related data were presented as mean±SD or median (minimum-maximum), whereas categorical variables were presented as frequency distribution and percentages. Qualitative data were analysed by using 2 / Fisher’s exact test to establish the association.
The receiver operating characteristic curve analysis was performed on the timing of tracheostomy and mortality to establish the cut-off point (days) for the early and late tracheostomy (Figure 2).
Youden’s index was calculated to determine the optimal cut-off point of the timing of tracheostomy, which was also represented by a sensitivity and specificity plot (Figure 3).
Predictors of 0- to 23-year mortality were identified by univariate cox proportional hazards analysis initially performed on candidate variables (including the timing of tracheostomy as a potential explanatory variable). Stepwise multivariable Cox regression analysis was performed on the clinically important variables and the independent variables having the probability of <0.15 in univariate analysis.
Mortality rates were calculated depending on the total number of years of follow-up for each patient. The survival probability with 95% confidence intervals was reported at various time intervals with the Kaplan-Meier technique (Figure 4).
Study population
The median age of patients at operation was 4 months (IQR: 23 days-9 months). Twenty-five (29.8%) patients were younger than 1 month, and 59 (70.2%) were between 1 and 12 months. Thirty (35.7%) infants in this study population were premature and thirty-nine (46.4%) weighed less than 50th percentile of predicted weight by national standards. [21] Twenty-nine (34.5%) patients required preoperative ventilator support. A combination of three or more risk factors for requiring prolonged mechanical ventilation was present in 58.3% (n=49) of patients (Tables 1-4).
A total of 10 (11.9%) patients had a genetic syndrome. Common genetic syndromes included trisomy 21 (n=3), DiGeoerge syndrome (n=2), congenital rubella syndrome (n=2), Pierre Robin syndrome (Glossoptosis, n=1), and VACTERL (vertebral abnormalities, anal atresia, cardiac abnormalities, tracheoesophageal fistula and/or esophageal atresia, renal agenesis and dysplasia, and limb defects, n=2). These patients had significant neurodevelopmental impairment in combination with chromosomal abnormalities. The underlying cardiac diagnoses for patients requiring tracheostomy are shown in tables 1 and E1 (Definitions).
In this study, 64 (76.2%) patients underwent tracheostomy between 10th-15th postoperative day and 20 (23.8%) patients underwent tracheostomy between 15-30 days following “primary operation” (Video Presentation).
In order to understand/analyze the outcome difference between early and late tracheostomy, the preoperative (pre-tracheostomy) status difference between two groups were analyzed. Descriptive characteristics and underlying cardiac diagnoses are summarized in table 1. The groups did not differ significantly in regard to age, sex, low birth weight, genetic syndrome, upper airway problems, preoperative lung infection/lobar collapse, sepsis, central neurological event, presence of excessive pulmonary secretions, poor systemic ventricular function, and unstable hemodynamics on preoperative inotropes (Table 5).
Short-term outcomes
There were 9 (10.8%) perioperative deaths after tracheostomy while still ventilated through the tracheostomy within 30 days following surgery. A combination of three or more risk factors was responsible for death in all patients. In 7 neonates, the causes of mortality were prematurity with low birth weight requiring preoperative ventilator support, lung collapse and sepsis who developed renal failure requiring peritoneal dialysis/hemodialysis and ultimately died of multiorgan dysfunction. One patient died of diffusely hypoxic cerebral injury and another patient died of new onset postoperative intracranial haemorrhage and cerebral coning.
Discharge mortality (2.4% vs 8.3%, p <0.001) and cardiac morbidity (11.9% vs 22.6%, p <0.001) was lower in the early tracheostomy group. Early tracheostomy was associated with decreased duration of mechanical ventilation (13±11 vs 31±15 days, p<0.001), ICU length of stay (18±12 vs 42±25 days, p<0.001) and decreased time of decannulation (14±9 vs 29±14, p<0.001) days. The overall postoperative sepsis was lower in the early tracheostomy group (7.1% vs 15.4%, p<0.001). There were no cases of mediastinitis in either group (Table 6).
There were significant differences between groups in regard to prematurity (p=0.03), preoperative ventilation (p=0.02), preoperative lung infection (p=0.001), renal dysfunction requiring peritoneal/hemodialysis (p<0.001), requirement of postoperative extracorporeal membrane oxygenation (p=0.012), delayed sternal closure/open sternum (p=0.002), cerebral hemorrhage (p=0.02), unilateral diaphragmatic paralysis requiring diaphragmatic plication (p=0.003), postoperative unstable hemodynamics (p<0.001), prolonged aortic cross-clamp time (p=<0.001), and requirement of reoperation (p=0.003) (Table 5).
Short-term outcomes
There were 9 (10.8%) perioperative deaths after tracheostomy while still ventilated through the tracheostomy within 30 days following surgery. A combination of three or more risk factors was responsible for death in all patients. In 7 neonates, the causes of mortality were prematurity with low birth weight requiring preoperative ventilator support, lung collapse and sepsis who developed renal failure requiring peritoneal dialysis/hemodialysis and ultimately died of multiorgan dysfunction. One patient died of diffusely hypoxic cerebral injury and another patient died of new onset postoperative intracranial haemorrhage and cerebral coning.
Discharge mortality (2.4% vs 8.3%, p <0.001) and cardiac morbidity (11.9% vs 22.6%, p <0.001) was lower in the early tracheostomy group. Early tracheostomy was associated with decreased duration of mechanical ventilation (13±11 vs 31±15 days, p<0.001), ICU length of stay (18±12 vs 42±25 days, p<0.001) and decreased time of decannulation (14±9 vs 29±14, p<0.001) days. The overall postoperative sepsis was lower in the early tracheostomy group (7.1% vs 15.4%, p<0.001). There were no cases of mediastinitis in either group (Table 6).
Neonates, prematurity (<37 weeks), low birth weight (<2.5 kg), preoperative ventilation, tracheostomy performed beyond 15 days of cardiac surgery, postoperative new onset cerebral hemorrhage, hypoxic cerebral injury, renal failure requiring peritoneal dialysis/hemodialysis, neonates requiring ECMO, LVEF (<0.40) and postoperative sepsis were significant negative factors for survival according to univariate analysis (Table 4).
Multivariate analysis identified six predictors for death after tracheostomy in this study group. The risk of death was 5.26 times higher (95% CI: 1.47, 20.36) in infants undergoing late tracheostomy (beyond 15 days) as compared to early tracheostomy (p=0.001). Neonates with renal failure requiring peritoneal dialysis/hemodialysis also exhibited 19.37 times (2.22-168.37) higher risk of death following surgery (p=0.007) (Table 7).
Long-term outcomes
There were 2 (2.7%) late deaths 39 and 81 months after surgery due to renal failure (n=1) and cerebral thrombosis (n=1) respectively. One patient was lost to follow-up. Seventy-two patients (98.6%) were followed up for a period of 1-276 month and yielded 885.6 patient-years of data. At a median follow-up of 166 (IQR: 82.5-116) months, the actuarial survival was 86.61% (SE± 0.04%; 95% CI: 77.1, 92.3). The actuarial survival was significantly lower in patients undergoing late tracheostomy (log rank p<0.0001), (Figure 4).
Cohort of survivors
At the completion of the period of review, 73 children were known to be alive. Late follow-up data was not available for one patient. All survivors (n=72) were examined and studied between June 2019 and December 2019, which was the closing interval of the study. Postoperative evaluation consisted of six monthly clinical examination, electrocardiogram, chest radiograph, soft tissue x-ray of the neck and echocardiogram. The severity of congestive heart failure was assessed according to Ross clinical score. [22]
Preoperative respiratory insufficiency was present in 55 (65.5%) of 84 infants, of whom 29 (34.5%) were already intubated and ventilated, and the remainder were not intubated but were oxygen dependent. The median total duration of mechanical ventilation before tracheostomy was 20 days (10-28 days). The interval between the primary operation and tracheostomy was 10 days in 23 (27.3%) infants, 15 days in 41 (48.8%) infants, and 30 days in 20 (23.8%) infants (Table 2).
Ventilation could not be weaned sufficiently to allow a trial of extubation in 39 (46.4%) patients. In 38 (45.2%) cases extubation had failed on single occasion and in 7 (8.3%) cases on two occasions. The reasons for prolonged ventilation were multifactorial for most patients. Cardiac cachexia, malnutrition, persistent postoperative pulmonary arterial hypertension, extremely poor body muscle reserves were other factors for requirement of prolonged ventilation in 39 (46.4%) infants (Table 3).
Neurological comorbidity was present in a total of 21 (25%) infants: six had experienced new insults, and five had pre-existing stroke, and 10 (11.9%) had major neurodevelopmental abnormalities. One infant had unilateral vocal cord palsy. Persistent chylothorax requiring prolonged intercostal drainage and reoperation affected four infants (Table 3).
Of the 84 children, 15 (17.8%) required cardiothoracic reoperations due to various causes. Two (2.4%) patients required revision of transannular patch. Two (2.4%) patients underwent tracheal operations for tracheal stenosis, 4 (4.7%) patients required ligation of the thoracic duct for chylothorax, and 7 (8.3%) patients required unilateral diaphragmatic plication (before tracheostomy).
Decannulation was successful in all 72 infants. The median duration of mechanical ventilation after tracheostomy was 22 days (range 11-35 days) and the median total duration of ventilator support (before and after tracheostomy) was 37 days (20-48 days). The median duration of tracheostomy to decannulation in survivors was 31 days (30-52 days). Sixty-eight (94.4%) survivors were in Ross’s clinical score of 2 and without antifailure cardiac medications.22 There were no reoperations during this period.
Complications of tracheostomy
One infant with tracheobronchomalacia had local infection at the tracheostomy site and the tracheostomy was exchanged for a period of nasotracheal ventilation. After the infection had cleared, the tracheostomy wound was revised without any further complications. Two children with subglottic stenosis who were preoperatively ventilated for 15 and 20 days respectively underwent excision of subglottic membrane and cricoid splinting and were ventilated for a period of 3 weeks in two sessions. There were no other complications, directly related to tracheostomy.
Prolonged tracheostomy ventilation
Preoperative ventilation before the “primary operation”, preoperative respiratory insufficiency, subglottic stenosis, tracheobronchomalacia, diaphragmatic paralysis, cardiac cachexia, and sepsis were associated with a longer period of tracheostomy ventilation. At the end of the follow-up, all survivors were successfully decannulated.
Analysis of the ROC curve, sensitivity and specificity plot
Tracheostomy performed between 12th and 14th postoperative day was associated with 100% sensitivity and 10.9% specificity in terms of superior clinical outcome. The timing of tracheostomy of 15 days as the optimal cut-off point was associated with a sensitivity of 73% and specificity of 84%, and at this time point, the Youden’s index was 0.60. The results obtained from the area under the ROC curve indicate that 83% (SE ±0.083, 95% CI 67.4, 1.0) of the time, the clinical outcome of postoperative patients was superior when tracheostomy was performed between 12th and 15th postoperative days compared with those performed between 15th and 30th postoperative days. Analysis of the sensitivity and specificity plot revealed the timing of the tracheostomy with an optimal cut-off point <16 days, Youden’s value of 0.64 and was associated with a sensitivity of 73% and specificity of 91% (Figures 2, 3).
Indications
The need for prolonged mechanical ventilation was a result of multifactorial etiology in over half of our patient cohort (49, 58.3%), most common being suboptimal hemodynamics due to cardiac cause in conjunction with renal failure requiring peritoneal dialysis and sepsis. On individual basis, the leading causative factors were cardiac cachexia (46.4%), severe cardiac-related pulmonary arterial hypertension (46.4%), followed by unstable hemodynamics (41.6%), persistent pulmonary infection, lung collapse & sepsis (22.6%), renal failure requiring peritoneal dialysis/hemodialysis (20.2%), and craniofacial syndromes with upper airway problems (19%) (Table 2). A number of studies found no association between surgical complexity and cardiac diagnoses with extubation failure. [1-11,18,19] However, other studies reported a history of pulmonary hypertension, prolonged CPB duration, deep hypothermic circulatory arrest, chromosomal abnormalities, and a high dose of narcotics as risk factors for extubation failure. [1-11,18,19]
Tracheobronchomalacia has been reported in 4% to 12% of young infants with CHD. [23-25] Additionally, it is not uncommon for patients with CHD to have a dysmorphic upper airway and genetic disorders. In this study, one infant had tracheobronchomalacia and two infants had subglottic stenosis requiring excision of the subglottic membrane. Although our patient cohort was not specifically evaluated for ciliary dysfunctional status, it remains an important consideration in syndromic patients. [26] Although not demonstrated in our study group, children with CHD might be at high-risk for airway compression by virtue of the anatomic proximity of cardiac chambers and great vessels to the central airways. [1-10]
Diaphragmatic paresis/ paralysis is less well tolerated in infants and small children due to relative weakness of the intercostal muscles, greater compliance of the chest wall, horizontal orientation of the rib cage, and increased mediastinal mobility with shifting of the mediastinal contents to the contralateral side on inspiration. Additionally, the recumbent position of infants reduces the vital capacity and small calibre of the infant bronchial tree, facilitates retention of secretions and bronchial obstructive debris. [27,28] Unilateral diaphragmatic paralysis reduces pulmonary function by about 25% in older children and by up to 60% in infants. [27,28]
The causative factors include intraoperative use of iced saline, cautery-induced neuropraxia, neurotemesis, surgical dissection close to the phrenic nerve, and staged palliation for univentricular repairs. The reported prevalence in retrospective studies varies from 0.3% to 5.7%. [27,28] In prospective studies, the reported incidence varies from 1.9% to 23.8%, which may indicate increased surveillance in prospective studies to detect asymptomatic cases. [27,28] In our cohort, 7 (8.3%) patients underwent plication for unilateral diaphragmatic paresis, however, it was not a significant factor in mortality. The opinion on timing of diaphragmatic plication varies from immediate plication on diagnosis, to a waiting period of 1-6 weeks in anticipation of spontaneous recovery. [27,28] In general, an arbitrary figure of 2-3 weeks of symptomatic diaphragmatic paralysis is advocated for diaphragmatic plication. [27,28]
Outcomes
Children requiring tracheostomy after surgery for CHD are at significant risk of poor outcomes with less than half still alive at a median follow-up of 3.9 years. [2-11] Mastropietro analysed 606 tracheostomies in neonates and infants in the STS Congenital Heart Surgery database in 2016 and found an overall mortality rate of 25.2%. [6] Johnson and associates in a multi-institutional study on 1292 tracheostomies in children undergoing cardiac surgeries reported an overall mortality rate of 21.6% (range 0% to 50%). [1]
Our overall mortality of 10.7% are in accordance with the published investigations which documents a tracheostomy-related mortality rate between 3.2% and 25% after 1985. [1-11] If a patient could not be extubated, we tried to identify anatomic or physiologic causes that were potentially reversible, e.g. plication of a paralysed diaphragm, optimization of hemodynamics with inotropic support, and nutritional supplementation to alleviate chronic cardiac cachexia. The decision of tracheostomy was taken before attempting second extubation in 45.2 % (n=38) of infants, and following failed second extubation in 8.3 % (n=7) of infants. Ventilation could not be weaned sufficiently to allow a trial of extubation in 39 (46.4%) infants.
In our study, logistic regression analysis accounting for the effects of other factors demonstrated neonatal intervention, ventilation requirement in the preoperative period because of unstable hemodynamics, renal failure requiring peritoneal/ hemodialysis, postoperative sepsis, postoperative new-onset cerebral hemorrhage, hypoxic cerebral injury as significant negative factors for survival. The risk of death was 5.26 times higher (95% CI: 1.47, 20.36) in infants undergoing tracheostomy beyond 15 days following surgery in comparison to those undergoing tracheostomy between 10 and 15 days following surgery (Table 7). All of our patients underwent open surgical procedure for tracheostomy. In this study, a midline vertical incision through the 2nd to 4th tracheal cartilages was used in all cases, thus minimizing incision of tracheal rings as few as possible.
Stomaplasty techniques described by Eliacher, Koltai and Bjork involves resection of tracheal cartilages and are recommended when longer duration of tracheostomy is expected.[17,29,30] We did not use any of these methods, since we expected our patients to be decannulated before discharge from hospital.
The reported incidence of tracheostomy-related complication is around 15% in adults and 15-19% in children. [1-11] Tracheitis was the most common complication in the reported literature followed by tracheostomy site breakdown. Other complications include tracheo-brachiocephalic artery fistulization, and mediastinitis. [31-35] Except for one infant who developed local infection at the site of tracheostomy, which was managed conservatively, and two children with subglottic stenosis, no other patients developed tracheostomy-related complications. We have a dedicated multidisciplinary team comprising of intensivists, tracheostomy nurses, chest physicians, ENT surgeons who carry out regular reassessments of all these patients while inpatient and after discharge. In our study population, we have been able to decannulate all infants prior to discharge. Due to lack of health care resources, hospital discharge with a tracheostomy or ventilator is not a viable option in India, therefore in-hospital decannulation is preferable in our setup.
We were particularly encouraged to find that there were no cases of mediastinitis in our group. Published reports on the incidence of mediastinitis following tracheostomy are varied from nil to significant risk by different investigators. [1-11,31-35] Some authors have demonstrated an increased risk of mediastinitis with tracheostomy following sternotomy in adult patients. [31,34] Percutaneous tracheostomy has been incriminated as a risk factor for mediastinitis by some investigators. [31,34,35] Two (2.4%) patients in this study developed spontaneous pneumothorax immediately following decannulation of the tracheostomy tube requiring intercostal drainage.
Five (6.8%) patients had superficial wound infection, which was managed conservatively. In case of clinical (fever, superficial wound infection, chest findings), laboratory (leukocytosis, elevated C-reative protein, procalcitonin), or radiological evidence (pneumonia), we begin broad spectrum antibiotics, and switch over to culture directed therapy on an individualized basis later on. The antibiotics were stopped when these features resolved.
Following placement of the tracheostomy tube, one of the concerns is the duration of time that the patient remains dependent on the device. In our study, all our patients were successfully weaned from positive pressure ventilation at a variable time period. Patients undergoing early tracheostomy were decannulated earlier than those undergoing late tracheostomy (mean, 14±9 vs 29±14 days; p<0.001).
Comparing outcomes in early and late tracheostomy
There is no consensus in the published literature on the indications and the duration a child should remain endotracheally intubated prior to tracheostomy and on the optimal pediatric decannulation protocol. [1-10]
Tracheostomy performed between 12th and 14th postoperative day was associated with 100% sensitivity and 10.9% specificity in terms of superior clinical outcome using the timing of tracheostomy of 15 days as the optimal cut-off point was associated with a sensitivity of 73% and specificity of 84%, and at this time point, the Youden’s index was 0.60. The results obtained from the area analysis of ROC curve indicate that 83% of standard error (SE) ±0.083, 95% CI 67.4, 1.0) of the time, the clinical outcome postoperative patients was superior when tracheostomy was performed between 12th and 15th postoperative days compared with those performed between 15th and 30th postoperative days.
In this study, 22.6% (n=19) had postoperative sepsis and was associated with 6.72 times (1.74, 25.92) increased risk of death on logistic regression analysis (Table 7). We believe that early tracheostomy leads to a decreased need for sedation, allows a gradual increase in the volume of enteral/parenteral feeds, thus improving nutrition, allows gradual weaning of ventilatory support, improves hemodynamics, shortens ICU stay and improves pulmonary toilet. [1-11]
In our cohort, ICU mortality and cardiac morbidity was significantly lower in the early tracheostomy group. Early tracheostomy was associated with decreased duration of mechanical ventilation, length of ICU stay, and decreased time of decannulation days. The overall postoperative sepsis was lower in the early tracheostomy group. There were no cases of mediastinitis in either group. However, adverse outcomes like sepsis, low cardiac output syndrome/suboptimal hemodynamics, delayed sternal closure, cardiothoracic reoperations were responsible for delaying tracheostomy placement, and thus more likely the plausible reasons for the differences seen in mortality.
Study Limitations
This study has several limitations. The study spans 23 years over which almost all aspects of intensive care have changed. Given its retrospective design, there may have been unmeasured confounding factors that influenced the observed outcomes and a prospective randomized trial of tracheostomy timing would be challenging given the small number of pediatric cardiac surgical patients who require prolonged ventilation, the diversity of diagnoses, and associated congenital and developmental factors. Short of randomization, propensity matching is probably the best protection against confounding and selection bias. Despite this, there may be confounding factors even propensity matching cannot overcome.
As this was a single surgeon study, it may not be possible to directly extrapolate the reported findings to other clinical settings. For example, the ventilation weaning protocols and extubation readiness test can vary among ICUs. Center-to-center variations in indications for tracheostomy, the timing of tracheostomy, and post tracheostomy care could potentially influence the outcomes. The small number of patients in this study, and the large number of candidate predictors analyzed in this study also limit the validity of results.
The study institution likely has a referral bias for high-risk patients with complex CHD, prematurity, late presentation, a referral from outside the geographic area. Additionally, a large proportion of the group having biventricular or univentricular repair had genetic syndromes or significant noncardiac comorbidities, which may reflect referral bias as well. Despite the limitations as above, the prevalence of extubation failure and the airway diseases in this study were comparable with recently published studies including high-risk cardiac surgical patients. However, our outcomes and possible risk factors need to be confirmed by larger multicenter studies with further investigation.
Our study demonstrates the relatively high incidence and diverse etiologies of extubation failure in pediatric cardiac surgery. We conclude that tracheostomy in neonates and infants undergoing cardiac surgeries, requiring prolonged mechanical ventilation provide a stable airway with improved pulmonary toilet, secures airway control, allows patient mobilization, allows the adequate intake of nutrients, thus improving cardiac cachexia and body mass, reduces work of breathing and allows gradual weaning from ventilation.
The timing of tracheostomy placement as it relates to the overall duration of positive pressure ventilation and survival between 10-15 days after surgery with the partially healed wound and mediastinal tissues appears safe. Early tracheostomy within 15th postoperative day was associated with decreased ICU mortality, decreased morbidity, and decreased ICU stay compared with tracheostomy between 15 and 30 days.
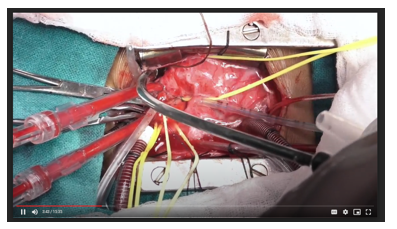
A presentation of an alternative technique for transfer of anomalous origin of left coronary artery from the pulmonary trunk in a child using autogenous aortic and pulmonary arterial flaps who underwent subsequent tracheostomy.36
Surgical planning and position
Following median sternotomy, the thymus was subtotally excised taking care not to expose the brachiocephalic vein. The pericardium was harvested using scissors about 1 cm infront of and parallel to the phrenic nerve, to avoid inadvertent cautery-induced ventricular fibrillation. Note the grossly dilated and tortuous coronary arteries. A continuous thrill is palpable at the root of the pulmonary trunk.
The operation was performed under moderately hypothermic cardiopulmonary bypass at 28°C using an angle venous cannula into the superior caval vein, a straight venous cannula into the inferior caval vein, and distal ascending aortic cannulation. Measures were taken to avoid excessive manipulation.
Dissection of the ascending aorta and pulmonary trunk
The pericardial reflection between the ascending and the pulmonary trunk was incised. The ascending aorta and pulmonary trunk were separated from each other. The persistent ductus arteriosus was interrupted using a liga clip (Johnson and Johnson Ltd., Ethicon, LLC, San Lorenzo, USA). The right and left pulmonary arteries were dissected and freed upto their first lobar branch.
Cross-clamping of the ascending aorta and pulmonary trunk, and administration of the cardioplegia
The aorta and pulmonary trunk were individually cross-clamped and cold hyperkalemic blood cardioplegic solution was infused simultaneously into both vessels at a pressure of 80 mmHg for 3 minutes to achieve optimal myocardial protection. This technique avoids run-off of the cardioplegic solution from the orifice of the proximal pulmonary trunk. A small right atriotomy was done for decompression of the right heart chambers due to the infused cardioplegic solution. The left heart was vented through the right superior pulmonary vein using a left heart DLP vent catheter (Medtronic Inc., Minneapolis, MN).
Transection of the distal pulmonary trunk and creation of a vertical pulmonary arterial flap
The pulmonary trunk was opened transversely in between stay sutures just below the bifurcation and completely transected. A DLP vent catheter was inserted into the distal end of the transected pulmonary artery to prevent flooding of the surgical field. A vertical flap of the pulmonary arterial wall was fashioned for preparation of a long arterial conduit to augment the bridging aortic flap to the anomalous coronary artery. Note the orifice of the left coronary artery posterolaterally within the left posterior aortic sinus.
The vertical pulmonary arterial flap of the sinus of valsalva containing the left coronary artery at its buttom was mobilized avoiding injury to the pulmonary valve leaflets. The proximal part of the left coronary artery was mobilized over a distance of about 1 cm.
Creation of an anteriorly based aortic wall flap
An arteriorly based, obliquely directed, rectangular flap of the aortic wall was created, approximately 10-15mm above the sinutubular junction, starting from the posterior aspect towards the left lateral side and continuing to the anterior aspect of the aorta, thereby maintaining anterior continuity of the aortic flap.
While selecting the site of re-implantation of the anomalous coronary artery, precautions were taken not to damage or distort the aortic valve, to implant the anomalous left coronary artery in the appropriate sinus, to elongate the main stem of the artery by combining the obliquely positioned rectangular aortic flap with a vertical pulmonary artery flap so as to obtain a “trapdoor” effect, and avoiding narrowing, flattening, torsion, tension or saccule formation, to direct the extended main stem of the anomalous artery posterior to the pulmonary trunk in anatomical position without extrinsic compression. The neo tunnelled left coronary artery was implanted above the sinotubular junction to ensure absence of kinking and external wasting of the left coronary without any tension.
Creation of an elongated neo-aortic tube by trap door technique
The isolated segment of the pulmonary trunk containing the left coronary artery at its bottom was approximated to the neoaortic window. The right inferomedial margin of the pulmonary artery flap was sutured along the posterior lip of the neoaortic orifice. The next point of suturing starts on the left inferolateral portion of the pulmonary arterial flap. The two adjacent edges of the inferolateral margin of the pulmonary arterial flaps were sutured with each other with left coronary artery orifice at its fulcrum.
Subsequently, the redundant superior edge of the pulmonary arterial flap was folded anteriorly over the left coronary sinus. Finally, the anteriorly based, obliquely directed, small rectangular aortic flap was sutured over the residual flap to obtain a “trapdoor” effect. Thus, an obliquely positioned extended left main stem was created, maintaining a tubular confiruation with the orifice of the left coronary artery at its fulcrum. Note the absence of narrowing, flattening, kinking, wasting, tension, torsion or saccular configuration of the neoaortic site.
Reconstruction of the pulmonary trunk
The aortic cross clamp was released, restoring myocardial perfusion. The pulmonary trunk was repaired on a beating, perfused heart. The defect created in the pulmonary trunk and its sinus was repaired with an autogenous pericardial patch using 6-0 monofilament polypropylene suture. Thus, a neoaortic conduit of viable, endothelialized, antogenous arterial flaps was created for transfer of the anomalous left coronary artery with correct angling and length, avoiding injury to the aortic and pulmonary valvular apparatus, without tension, torsion, traction, or saccular configuration, avoiding obstruction of right ventricular outflow tract
No potential conflicts exist
Source of funding: Nil
Informed consent and Patient identification release statement: Obtained from all patients
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.
Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Giselle Pentón-Rol.
