AUCTORES
Globalize your Research
research article | DOI: https://doi.org/10.31579/IJBR-2022/088
1Biochemistry Labs, Sherbin Central Hospital, Ad Daqahliyah, Ministry of Health, Egypt.
2Research and Development Department, Biotechnology Research Center, New Damietta, Egypt.
3Department of Chemistry, Faculty of Science, Port Said University, Egypt.
4Faculty of Pharmacy, Middle East University, Amman, Jordan.
5Genetics Unit, Faculty of Medicine, Mansoura University, Egypt.
6Department of Surgical Oncology, Oncology Centre, Faculty of Medicine, Mansoura University, Mansoura, Egypt.
*Corresponding Author: Mohamed A. Abdelrazek. Biochemistry Labs, Sherbin Central Hospital, Ad Daqahliyah, Ministry of Health, Egypt.
Citation: Mohamed A. Abdelrazek, Ahmed Nageb, Mohamed J. Saadh, Rizk Elbaz, Amr Abouzid, Lamiaa A. Barakat. (2022). Serum TFF3 in breast cancer development and progression. International J. of Biomed Research. 2(8): DOI: 10.31579/IJBR-2022/088
Copyright: © 2022, Mohamed A. Abdelrazek, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 25 July 2022 | Accepted: 01 August 2022 | Published: 10 August 2022
Keywords: Breast cancer; Diagnosis; Biomarker; TFF3; Serum
Background: Searching for new methods for breast cancer (BC) diagnosis at early stages is widely demanded. Trefoil factor-3 (TFF3) mRNA is expressed in BCs and cell lines. We aimed in this study to evaluate TFF3 clinical value in BC development and progression.
Methods: In Egyptian females with BC (n=120), benign breast disease (n=40) and healthy women (n=40), TFF3 blood levels were measured by ELISA. Receiver operating characteristic (ROC) curve was performed for evaluation TFF3 diagnostic ability.
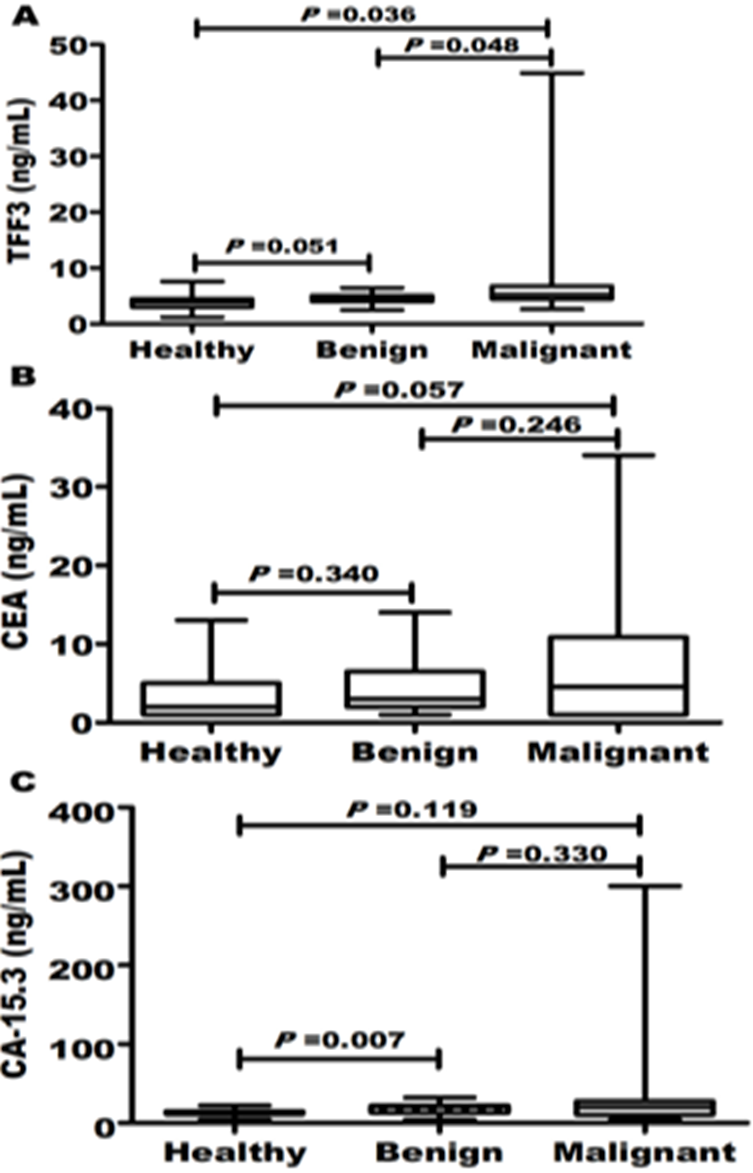
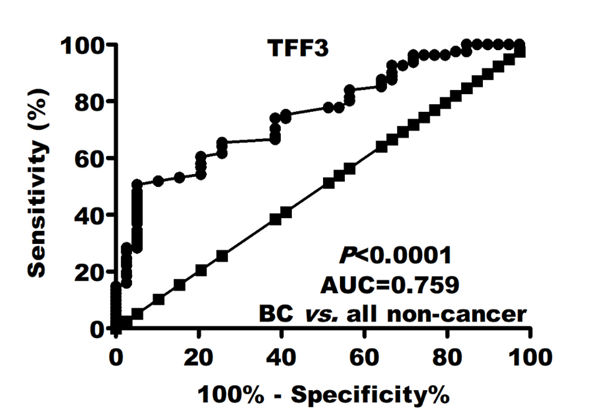
Results: Serum TFF3 was more significantly (P=0.027) higher in BC patients [5.3 (4.5-6.7) vs. 4.7 (4-4.8) and 3.9 (3-4.4)] than benign and healthy controls, respectively. TFF3 (AUC=0.759; P<0.0001) had superior diagnostic power compared to CEA (AUC=0.570) and CA-15.3 (AUC=0.619). TFF3 sensitivity and specificity were 75 and 60%, respectively. This good performance did not significantly affect in disease early stages (AUC=0.718; sensitivity=71.8%; specificity=60%). This diagnostic power increase when comparing BC patients to only healthy controls (AUC=0.840; sensitivity=75%; specificity=77.5%). Elevated TFF3 levels were associated disease progression including tumor multiple lesions (r=0.23), late stage (r=0.25), high grade (r=0.34), large size (r=0.28), lymph node involvement (r=0.33) and non-luminal BC molecular subtypes.
Conclusions: TFF3 is inexpensive, rapid, easy to perform and reliably BC biomarker. Moreover, its association with disease severity may support its potential role as prognostic BC marker.
[1]. Despite a lower BC incidence in developing countries, the mortality rate is generally higher compared to developed countries [2]. This is likely due to limited access to treatment, late stage at diagnosis and delayed disease presentation. This shows both their lifestyles BC risk factors and the utility and availability of proper BC screening [3]. From another hand, effective treatments are suitable for patients with early stages instead of patients with late stages [4]. Thus, identifying easy and specific biomarkers associated with BC development and progression is vital for improving BC patients prognosis efficacy [4].
Trefoil factors are stable small peptides that secreted by gastrointestinal tract epithelial mucus secreting cells [5]. They constitute 3 peptides (TFF1, 2, and 3) that are broadly expressed in tissue specific manner [5]. Human TFF3 gene encodes small secreted protein (59 amino acids) [6]. Increasing evidence has suggested that TFF3 has important roles in both development and progression of several human tumors [6]. Indeed, TFF3 expression was reported to be increased in prostate [7], mammary [8], lung [9], hepatocellular [10], gastric [11], endometrial [12] and colorectal [13] carcinomas. In contrast to normal mammary gland [14], elevated TFF3 expression was reported in BC tissues [15] and these levels were positive in more than 90% of invasive ductal carcinoma [16].
As studies evaluating TFF3 as BC diagnostic biomarker and also these that regarding its association with BC clinicopathological features are very limited, we aimed in this study to further evaluate TFF3 BC diagnostic value compared to established BC tumor markers (CEA and CA-15.3). Also, we aimed to evaluate its association with some tumor severity features including, number of lesions, stage, grade, size and lymph node invasion.
Study population
Retrospective study was performed between February and June/2021 involving Egyptian women with BC (n=120), with benign diseases (n=40) and 40 age-matched healthy females. They were recruited from Oncology Centre, Mansoura University, Egypt. All patients were clinically, radiologically and pathologically diagnosed for BC. All patients were hormonally healthy and patient with metastases, other tumor types or undergone radio-/chemo- therapy were excluded. None of healthy and benign controls had any malignant history. The disease was pathologically classified according to Tumor-Nodes-Metastasis (TNM) Classification of Malignant Tumours of the Union for International Cancer Control [17]. Study protcol was approved by Mansoura University Hospitals Ethics and Scientifc Committees. Written informed consent was obtained from all patients and controls.
Biochemical analysis of tumor markers
After diagnosis and according to the manufacturer’s instructions, TFF3 was determined using ELISA commercial kit (Abcam, Cambridge, UK). Also, all patients and controls were screened for CEA and CA-15.3 using commercial ELISA kit (MyBioSource, San Diego, USA).
Parameter | Healthy | Benign | Breast cancer | P value | |
Number | 40 | 40 | 120 | ـــ | |
Age (years) | 44.0 ± 8.6 | 46.4 ± 9.1 | 48.6 ± 10.9 | 0.553a | |
Menopause (Pre-/post-menopausal) | 18/22 | 16/24 | 43/77 | 0.455b | |
Lesion (single/multiple) | ـــ | ـــ | 96/24 | ـــ | |
Tumor size (<2cm>2cm) | ـــ | ـــ | 56/64 | ـــ | |
Tumor stage (T≤2/T>2) | ـــ | ـــ | 78/42 | ـــ | |
Tumor grade (G1/G2-3) | ـــ | ـــ | 36/84 | ـــ | |
Lymph node (negative/positive) | ـــ | ـــ | 60/80 | ـــ | |
Estrogen receptor (negative/positive) | ـــ | ـــ | 36/84 | ـــ | |
Progesterone receptor (negative/ positive) | ـــ | 33/87 | ـــ | ||
HER-2/neu (negative/positive) | ـــ | ـــ | 42/78 | ـــ | |
Differences between groups were established by a ANOVA or b Chi-squared (X2), appropriately.
Table 1: Characteristics of included females
Analyses were performed using GraphPad Prism and SPSS programs. Variables levels was expressed as absolute numbers, mean±SD or median (interquartile range), appropriately. ANOVA, Chi-squared (X2) or Kruskal-Wallis tests were appropriately performed for differences assessments follwed by LSD as post-hoc test. P value <0 n=200)>
TFF3 and breast cancer development
Patients and controls were age-matched (P=0.553). Most of included women were postmenopausal. Tumor features including stage, grade, size, lymph node status, hormonal status and HER-2 protein detection were summarized in Table 1. Despite CEA and CA-15.3, it was reported that serum TFF3 [5.3 (4.5-6.7) vs. 4.7 (4-4.8) and 3.9 (3-4.4)] in BC patients was more significantly (P=0.027) higher than benign and healthy controls, respectively (Figure 1).
TFF3 diagnostic ability
Regarding BC diagnosis, ROC curve analysis revealed that TFF3 (AUC=0.759; P<0>4.4 ng/mL, TFF3 sensitivity and specificity were 75 and 60%, respectively. In early stages detection, this good diagnostic performance did not significantly affect (Table 2). These values rose to AUC=0.862; sensitivity=73.3%; and specificity=77.5% when discriminate BC patients to only healthy controls.
Marker |
AUC (95% CI) | P value | Cutoff | Sensitivity (%) | Specificity (%) |
BC from all non-cancer | |||||
CEA (ng/mL) | 0.570 (0.47-0.67) | 0.229 | 2.6 | 61.7 | 50 |
CA-15.3 (ng/mL) | 0.619 (0.52-0.72) | 0.035 | 14.5 | 65 | 56.3 |
TFF3 (ng/mL) |
|
| 4.4 | 75 | 60 |
Early stages (T>2) from all non-cancer | 0.759 (0.69-0.85) | <0> | |||
CEA (ng/mL) | 0.547 (0.41-0.65) | 0.434 | 2.6 | 56.4 | 50 |
CA-15.3 (ng/mL) | 0.602 (0.50-0.70) | 0.087 | 14.5 | 61.5 | 56.3 |
TFF3 (ng/mL) |
|
| 4.4 | 71.8 | 60 |
BC from healthy | 0.729 (0.65-0.84) | <0> | |||
CEA (ng/mL) | 0.621 (0.50-0.74) | 0.111 | 2.6 | 61.7 | 56.3 |
CA-15.3 (ng/mL) | 0.690 (0.58-0.80) | 0.011 | 14.5 | 65 | 72.5 |
TFF3 (ng/mL) | 0.840 (0.75-0.93) | <0> | 4.4 | 75 | 77.5 |
Table 2: Diagnostic performance of HE4 to discriminate BC patients
Abbreviations: CEA: carcinoembryonic antigen; CA-15.3: cancer antigen 15.3 and TFF3: trefoil factor 3.
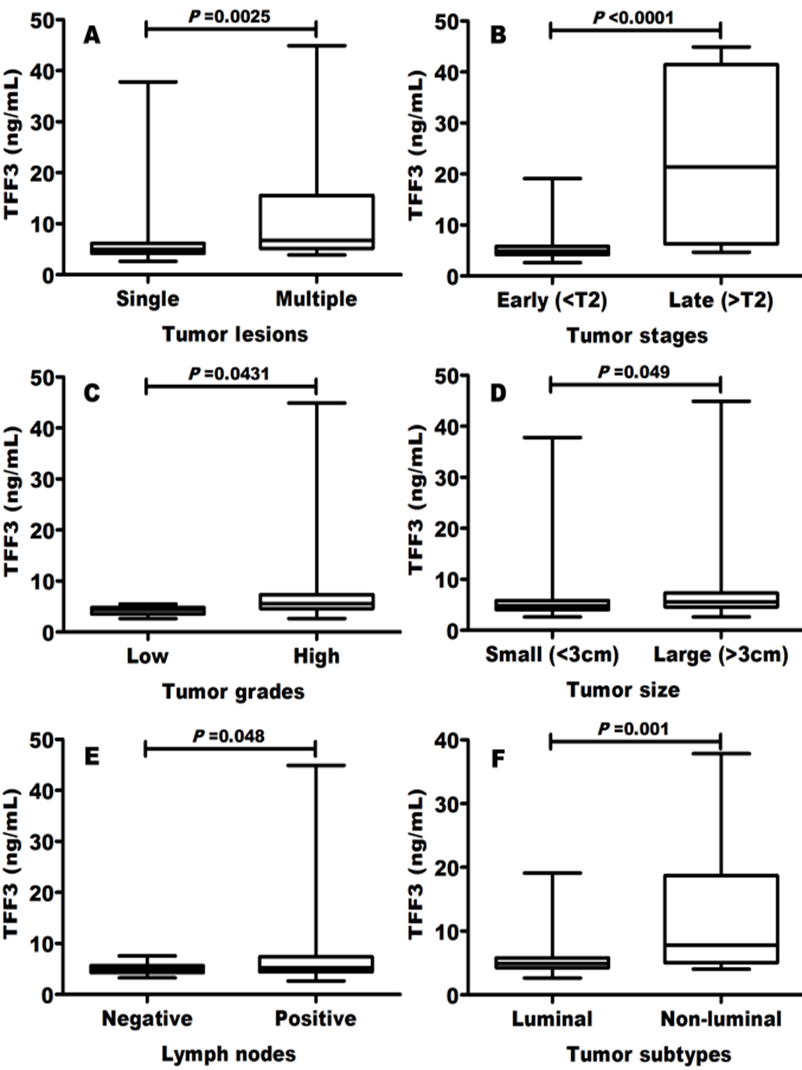
Elevated TFF3 was associated with disease progression
Elevated TFF3 blood levels were significantly (P<0>r=0.23), late stage (Figure 3B; r=0.25), high grade (Figure 3C; r=0.34), large size (Figure 3D; r=0.28) and lymph node invasion (Figure 3E; r=0.33). Moreover, TFF3 levels were greater in non-luminal compared to luminal BC molecular subtypes (Figure. 3F).
Factor | Spearman correlation | |
r | P value | |
Lesions | 0.23 | 0.039 |
Tumor T stage | 0.25 | 0.040 |
Tumor grade | 0.34 | 0.004 |
Lymph node | 0.33 | 0.045 |
Clinical stage | 0.30 | 0.018 |
Tumor size | 0.28 | 0.011 |
Table 3: Correlation between TFF3 and some tumor features
Understanding the diagnostic modalities and complex algorithms used to assess prognostic, predictive and diagnostic biomarkers is central for quality oncology care [19]. TFF peptides are family of growth factor-like peptides which was first suggeted in 1988 by Thim [20]. Their gene expression has been detected in almost all mucus-secreting cells, e.g., cervical secretions, seminal plasma, prostate, respiratory tissues, salivary glands, ocular tissues, gastrointestinal tract and milk [21]. Among them, TFF3 mRNA is expressed in BCs and cell lines [8]. This mRNA expression is related to osteo- and cerebrospinal- metastasis. Also, its protein expression is associated with local metastasis and lymph node involvement [8].
In this study, we aimed to evaluate blood TFF3 easy test in BC detection using two controls group (healthy females and patients with benign breast disorders). Our results revealed that that serum TFF3 was more significantly (P=0.027) higher in BC patients [5.3 (4.5-6.7) vs. 4.7 (4-4.8) and 3.9 (3-4.4)] than benign and healthy controls, respectively. Moreover, ROC curve analysis showed that TFF3 (AUC=0.759; P<0 AUC=0.570) AUC=0.619). AUC=0.840; sensitivity=75%; specificity=77.5%).>
These results demonstrate that TFF3 may be a promising predictive BC biomarker. TFF3 is as an oncogene that may stimulate tumor cell oncogenicity, survival, proliferation, and invasion in several cancers, such as prostate, gastric and mammary carcinomas [22]. In both invasive BC and ductal carcinoma in situ, elevated TFF3 expression is observed [15, 23]. In mammary carcinoma, it was reported that TFF3 is potent angiogenic factor and functions as de novo angiogenesis promoter. This may co-coordinate with tumor growth, metastatic actions and enhancing tumor progression [24]. Similar to our findings, Ishibashi et al. immunohistochemically found that TFF3 expression was positive in 73.9% of BCs. They also found that serum TFF3 was significantly higher in BC patients and they found that TFF3 had AUC=0.72 [5].
Another important result of this study is that elevated TFF3 levels were significantly (P<0>r=0.23), late stage (r=0.25), high grade (r=0.34), large size (r=0.28) and lymph node involvement (r=0.33). Moreover, TFF3 levels were greater in non-luminal compared to luminal BC molecular subtypes.
It is well known that this peptide is related to tumor metastasis and invasion which plays very important roles in tumors progression [22]. In BC, TFF3 participated in tumor invasion metastasis through CDH1repression mediated by STAT3 [25]. CDH1, tumor suppressor glycoprotein, is one of main cell adhesion complexes constituents and mediates calcium-dependent cellular interactions in epithelial cells, which plays important role in adherent type junctions’ establishment [26]. CDH1 expression dysfunction or loss associates with cell–cell interaction loss which promotes tumor cells to gain an invasive cell phenotype and metastasis [22]. Our results found that elevated levels of TFF3 were associated with non-luminal tumor subtypes and this is in agreement with other studies that found that TFF3 expression is significantly associated with increased resistance to chemotherapy [27].
In conclusion in this study compared to benign and healthy controls, expression levels of TFF3 were significantly elevated in BC patients. In contrast to other established biomarkers (CEA and CA-15.3), TFF3 had superior diagnostic ability in BC detection especially in early disease stages. This study may include some limitations like retrospective nature and single-center patient’s cohort. Thus, future more multicentric comprehensive studies are required to examine TFF3 prospective analysis.
None
None