AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2643-6612/043
1 IMA MARIJNSKAYA academy, applied pharmacologist, hospital pharmacist manager, member of ITALIAN GALENIST UNION, Galenic laboratory PC AREA, italy 29121.
2 Professor of phisical chemistry, Libyan Authority for Scientific Research.
3 IMA Maijnskaya academy Italy.
4 Independent researcher, hospital pharmacist manager PC AREA.
5 Professor, Department of Medical & Health Sciences for Woman, Peoples University of Medical and Health Sciences for Women, Pakistan.
6 Medicinal chemist, Nano Drug Delivery, (a Product Development Firm), United States.
7 Bio-medical laboratory turin italy Citta’ della Salute.
8 IMA president RU.
*Corresponding Author: Luisetto M. Ima Marijnskaya academy, applied pharmacologist, hospital pharmacist manager, member of Italian Galenist Union, Galenic laboratory PC AREA, Italy 29121.
Citation: Luisetto M, Khaled Edbey, Riccardo Benzi Cipelli, Fiazza C, Mashori Gulam Rasool, et al, (2024), Non sterile clinical Galenic Laboratory : a scientific discipline Between laboratory practice clinical pharmacy and personalized pharmacological therapy. The semplified normative rules NBP in Italy, J Dentistry and Oral Maxillofacial Surgery, 6(1); DOI: 10.31579/2643-6612/043
Copyright: © 2024, Luisetto M. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 05 February 2024 | Accepted: 16 February 2024 | Published: 28 February 2024
Keywords: galenic laboratory; magistral formula; clinical pharmacy; pharmaceutical care; personalized pharmacy; medicine shortage; nbp; semplified rules; official pharmacopeia; control process; quality management system; rare disease; 3d printing systems
Observing the today hospital practice in many countries and the international literature involved it is clear How clinical pharmacy is linked to the galenic laboratory practice. Today more then recent past due to the various kind of magistral formula request by the clinicians It is necessary that the clinical pharmacist perpesctive must to be added to the classic GALENIC laboratory competencies: this make possible to complete the profile of efficacy and safety of this fundamental Drugs. The clinical galenic activity is divided in sterile and Non sterile. (Total parenteral nutrition bags, Pain therapy, oncological parenteral drugs laboratory, radio drugs and diagnostics, non sterile galenics). Aim of this work is to deeply investigate this crucial link (CLINICAL PHARMACY with LABORATORY PRACTICE) in order to get the really best clinical results for the patients. Clinical pharmacy principle, PHARMACEUTICAL care, managerial competencies and personalized pharmacy added to the best knowledge and competencies in galenic laboratory make the difference in order to obtain the right final clinical results. The same in this work are submitted to international pharmacy practioner, directors and researcher the Normatives rules operating in an advanced country: the semplified italian NBP, example that can be applied also in non advanced nations. In italy pharmacist can follow or the full NBP of the official pharmacopeia or the semplified according DM Salute 18.11.2003. ( related the kind of galenic formula if sterile or not)
The NBP (good manifacturing rules) introduce an QUALITY SYSTEM MANAGEMENT.
The full NBP are more used in more complex laboratory in example involved in specialistic products Like oncologic or radiopharmaceutical and other. Aim of this rules is to guarantee quality, security and efficacy of a drug prepared in galenic lab. This are based on resonsability principles, plan, documentation of all activity. (QUALITY SYSTEM OF ASSURANCE) All phases of the preparation are under the responsability of the pharmacist. The final quality depends on the correct use of API (ACTIVE PRINCIPLE) and eccipients, the right calculations operation, right volume or weight operation, and following the right procedure. Check on the final products: folowing of the procedure, aspetcs, pakaging and its closure. According NBP the laboratory must to be separated (or it must to be separable) form the pharmacy and a second pharmacist (that is different form the pharmacist that prepare ) must to check the final preparation. The locals must to be according striclty enviromental condition to make possible to prepare in safety way the drugs. And it is mandatory must to be followed written procedure. (the instrument verify, training of the pharmacist)Cleaning procedure, sanifications. Raw material certifications, technical sheet, safety sheet. Working sheet is mandatory. The pharmacist can follow this two option related the kind of drugs produced and the characteristic of the laboratory. It is not the main focus of this work to produce a literal translation of DM 18/11/03 only to submit its general meanings. This can be considered for the authors useful to be added also to the normative rules in force in non advanced countries.
Starting from the consideration that in history of the remedy fot the pathology of humans great contribute was obtained with the introduction of GALENIC principle and methods. From GALENUS form Pergamon (Greek) 129 dc – 201 comes the term GALENIC art of the pharmacist to produce drugs inside in the pharmacy.He codified the preparation of drugs using multiple kinds of ingredients. (active principle added with excipients). For many centuries this methods was used in the laboratory to produce remedy to treat many human pathology. Federico II Svevia 1194 – 1250 knowed as “ STUPOR MUNDI “ related his open mind concepts introduced In europe and in italy the need to have specific rules for regulation the activity of drugs production in the pharmacy laboratory from the prescription activity of the physicians.
This in order to avoid conflic of interest between this prescrictive funtion form the pharmacy practice:
this produced the mandatory separation between medicine and pharmacy.: to the phisicians the role in therapy and responsible for prescriptions of durgs and the pharmacyst responsible for production and sell of the drugs . But during the illuministic period , the industrial revolution , the succes of medicinal chemistry since 1800 and 1900 many forces make possible to shift the drugs production form the pharmacty to the more complex industry . During all this periods many FORMULARY and then PHARMACOPOEIA in various countries was introduced and adopted To make possible to get adeguate quality of the drugs produced, safety and reproducibility of the procedure. (monography, methods of analisys ,table et other )This texts becomes mandatory by healthcare law in the various contests ( FU italian, FU european, US pharmacopeia and many other examples).
Also the competencies of who was involved in remedy preparation increased during centuries :
from botanic experties (SCUOLA SALERNITANA VII e VIII century ) to the IATROCHEMISTRY principle PARACELSUS from XVI century ) . Before the pharmacists, apothecaries that worked alongside priests and physicians in regard to the patient care. The history of pharmaceutical history is well knowed form introduction of the first SULFAMIDICS since the Actual last antivirals (for covid-19 treatement). But, related the last industrial pharmaceutical revolution ,some problem arosed : not all prarmaceuticals industries produce drgus for all subpopulation (pediatric patients, swallowing problems in geriatrics). There is the needs of personalizaed dosages or personalized pharmaceutical form (for pediatric or geriatric patients) needs to introduce drugs in enteral nutrition drugs not available form national or foreign producers (national or international shortcomings)
orphan drugs for some rare disease
dermatologic products
cannabis preparates
some disifectants band antiseptics formula
some antidothes (galenics)
some laboratory reagents and solutions
some contrast agents
odontoiatric galenics
and many other So due to this failure of industry to cover all this situation the galenic laboratory is a real opportunity.Today also many pharmaceutical industry not like more to produce classic drugs as many cardioactive products and other and the magistral product make possible to overcome this problem. ( expcially today whit actual economic crisis) . Also a great number of galenic formula are in use commonly in the hospital : corrosive products for dermatologist, alcool solution for laboratory, various reactives , phitotherapic derivates and so on . Galenic Pharmacy also provides educational, scientific and research activities in the profile discipline – pharmaceutical technology to the pharmacy student or under specilization programs. But observing international literature it is possible to see that the best clinical resulats are obtained when the laboratory activity in production magistral formula by the phyicians is completed when available the clinical pharmacist and managerial competecies in the same team. Galenics is the laboratory process that turns an active ingredient (API) into a ready-to-use medicine that can be dosed as required for the various patients . This to optimise their absorption. It the discipline (or science) of dosage form design. According Review Braz. J. Pharm. Sci. 56 2020 https://doi.org/10.1590/s2175-97902019000418358 Preparation of extemporaneous oral liquid in the hospital pharmacy Márcio Robert Mattos da Silva Letícia Pereira Dysars Elisabete Pereira dos Santos E. Ricci Júnior “At the hospital, the pharmacist is constantly challenged to prepare extemporaneous solutions ES from tablets, capsules or drug powder for patients unable to swallow, Like as pediatric, elderly and patients that use nasoenteric and nasogastric tubes. The preparation of extemporaneous solutions ES from capsules, tablets and drug powder requires stability studies analysis”

Figure 1: manual encapsulator, an expample of instrument used in galenic laboratory

Figure n 2: galenic laboratory, an example of solution preparation by the pharmacist
With and observational point of view an review of relevant article ( references form 1-16 ) related the topics of this work is performed .
It is produced the meaning translation of an Italian normative rule DM 18 NOV 2003
An practical experimental study is reported with results from 2008 to 2023 .
Finally a global conclusioni is submitted to the researcher related also innovations in fields of Galenic laboratory.
Review RESULTS
FROM LITERATURE
J Pharm Pract. 2021 Jun 15;8971900211023643. doi: 10.1177/08971900211023643.
Hospital Pharmacy Response to Covid-19 Pandemic in Italy: What We Learned From the First Outbreak Wave
Vera Damuzzo , Riccardo Bertin , D. Mengato , M. Chiumente , Melania Rivano , Angelo Claudio Palozzo
DOI: 10.1177/08971900211023643
“ When COVID-19 pandemic started, the Italian hospital pharmacists faced multiple challenges and change their work practices.
The aim of this study work was to describe the impact of the C-19 emergency on pharmaceutical care (PC) provided by pharmacists during the first wave of the pandemic. Issues related to pharmacist's involvement in the pandemic management PM were: changes in activities, support received by authorities and pharmacists' own perceived role in the Health System HS .
A cross-sectional study worl based on a web survey was conducted between May and June 2020 collecting information from pharmacists, members of Italian Society of Clinical Pharmacy and Therapeutics SCPT. 113 (11.4%) completed the questionnaire. The cohort was divided in two arms: pharmacists who worked in severely C-19 affected areas (High Spread Regions) and those employed in the less affected areas (Low Spread Regions).
The changes in the pharmacy work settings PWS reflected the increase of logistics area and non-sterile clinical galenic, and reduction of clinical tasks.
The most demanding challenge was referred to shortages of medical devices MD and drugs, 61/113 pharmacists reported difficulty in obtaining products compliant to quality standards. National Institutions and the Regional Governments provided a greater perceived support. More than about 50% of participants felt that their role did not change if compared to other healthcare professionals.
Despite some limitations related to their clinical activity, pharmacists played a relevant l role in supplying personal protective equipment, medical devices MD and medications to improve health outcomes during this emergency. The results may guide pharmacists in future actions to improve the management of the pandemic. “(6)
Croat Med J. 2014 Dec; doi: 10.3325/cmj.2014.55.662
Establishment of galenic laboratories in developing countries to produce high quality medicines: results of Aid Progress Pharmacist Agreement (A.P.P.A.®) Project
Francesca Baratta, A. Germano, Gaetano Di Lascio, Richard Petieau,and Paola Brusa
“Aid Progress Pharmacist Agreement Project: aims in developing countries
Aid Progress Pharmacist Agreement (A.P.P.A.®) is a non-profit NP association based on a voluntary work and its main activity is the A.P.P.A.® Project. The Project started in the 2005 as a result of the cooperation between the Pharmacy Faculty of Turin (TO) and Italian community pharmacists. Its main task is the establishment of galenic laboratories (GLs) in hospitals of developing countries (DCs) according to the principles of international health cooperation.
- establishing GLs in DCs with the aim of preparing medicinal products MP that comply with quality requirements, first of all to fight the widespread counterfeiting of medicines in DCs;A
-tailoring the dosages and pharmaceutical forms PF according to the actual patient needs;
-employing the local staff, teaching them a “new job,” and opening a suitable school;
-minimizing the costs necessary to prepare these medicines.
There are relevant and important reasons why galenics should be used:
i) a low cost of the production system and simple operative procedures;
ii) the possibility to adapt the dosages and pharmaceutical forms PF to the patients’ needs and medical prescriptions;
iii) reduction in the use of counterfeit medicines CM in the settings where the GL is located. “(7)
Study protocol 08 January 2018
Impact of collaborative pharmaceutical care on in-patients’ medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study)
Géraldine Leguelinel-Blache, Christel Castelli, C.Roux-Marson, Sophie Bouvet, Sandrine Andrieu, Philippe Cestac, Rémy Collomp, Paul Landais, B. Loulière, Christelle Mouchoux, Rémi Varin, Benoit Allenet, MEDREV Working Group, Pierrick Bedouch & Jean-Marie Kinowski
“The clinical pharmacist CP will have a collaborative meeting with both the prescriber and the nurse in order to notify any possible medication errors ME and suggest any proposals to optimize the AMO according to the medical history, the clinical status CS , and the therapeutic adherence . ( change of galenic form due to swallowing problem, dose adjustment to renal function RF ). After the collaborative meeting, the clinical pharmacist will check whether the prescriber has accepted his/her suggestion(s) and modified the AMO. All the pharmaceutical interventions, the medication errors ME detected and the pharmaceutical suggestions of order modification, will be collected and characterized in a standardized form according to the French Society of Clinical Pharmacy FSCP “ (8) JDDG: Journal der Deutschen Dermatologischen Gesellschaft Topical preparations and their use in dermatology J. Wohlrab 23 November 2016 https://doi.org/10.1111/ddg.13151 “The choice of a pharmaceutical (galenic) concept is primarily based on the requirements of the physico-chemical properties PCP of the active ingredient to be applied. The fixed combination of active pharmaceutical ingredients API in topical preparations is suitable for only a limited number of clinical treatment scenarios.” (9) Hospital Pharmacology. 2015; Information on the Quality of Substance for the Preparation of Pharmaceutical Drugs in Terms of Hospital Pharmacy M. Dj. Jovović , Maja M. Ribar “Compliance with national legislation, like as establishing compliance prescribed by the European legislation EL in the field of drug development is binding. All manufacturers of drugs and/or active pharmaceutical ingredients PI must apply quality standards prescribed by the European Pharmacopoeia EP in order to develop, manufacture and sales of medicines. When it comes to the quality of pharmaceutical ingredients PI for the production of drugs in the pharmacy, pharmacies especially in residential institutions in our country is permanently done by harmonizing national legislation NL in order to improve conditions for the preparation and production of galenic drugs GD in terms of inpatient health institutions performed in a manner that is prescribed by international regulations. Th is requires the adaptation of institutions, including the fundamental changes in competence as national professional and administrative and regulatory rules that apply to state- and private sectors “(10)
ORIGINAL ARTICLE
DOI: 10.1016/j.rppede.2016.02.012 Magistral drugs in hospitalized newborns and childrenMedicamentos magistrais em recém-nascidos e crianças hospitalizados Agueda Cabral de Souza Pereira, Elaine Silva Miranda, S. Rodrigues de Castilho, Débora Omena Futuro, Lenise Arneiro Teixeira, Geraldo Renato de Paula Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil “The constant consumption of magistral oral solutions MOS and suspensions by newborns and children of the assessed hospital indicates the need for such preparations as a pediatric therapeutic alternative in this hospital.”(11)
Luisetto, M et al (2017). Editorial The Clinical Pharmacists Main Focus Journal of Applied Pharmacy, 2017, Vol.09(04)
Mauro LUISETTO et al (2015). UK Journal of Pharmaceutical and Biosciences Vol. 3(6), 67-72, 2015 RESEARCH ARTICLE Pharmacist Cognitive Service and Pharmaceutical Care: Today and Tomorrow Outlook
Fiazza C, Ferraiuolo A,Luisetto M, Sahu R. (2020).Galenic hospital laboratory during COVID-19 emergency: A practical experience in an advanced country. Int J Clin Virol.; 4: 118-125.
Mauro Luisetto,(2017).Attitudes and Skills in Business Working Settings: A HR Management Tool Business and Economics Journal Mauro, Bus Eco J 2017, 8:1
m.luisetto et al (2016).management instrument in pharmaceutical care and clinical pharmacy Intern. journal of economics and management sciences Luisetto et al., Int J Econ Manag Sci 2016, 5:5
Vera Damuzzo , Riccardo Bertin , Daniele Mengato , Marco Chiumente , Melania Rivano , Angelo Claudio Palozzo (2021).J Pharm Pract. 2021 Jun 15;8971900211023643. Online ahead of print. Hospital Pharmacy Response to Covid-19 Pandemic in Italy: What We Learned From the First Outbreak Wave PMID: 34126804
Francesca Baratta, Antonio Germano, Gaetano Di Lascio, Richard Petieau, and Paola Brusa(2014) .Establishment of galenic laboratories in developing countries to produce high quality medicines: results of Aid Progress Pharmacist Agreement Journal List Croat Med J v.55(6); 2014 Dec PMC4295074 Croat Med J. 2014 Dec; 55(6): 662–668. (A.P.P.A.®) Project
Géraldine Leguelinel-Blache, Christel Castelli, Clarisse Roux-Marson, Sophie Bouvet, et.al (2018).Study protocol Open Access Published: 08 January 2018 Impact of collaborative pharmaceutical care on in-patients’ medication safety: study protocol for a stepped wedge cluster randomized trial (MEDREV study) Trials volume 19, Article number: 19 (2018)
Johannes Wohlrab (2015), Topical preparations and their use in dermatology JDDG: Journal der Deutschen Dermatologischen Gesellschaft First published: 23 November 2016
Marija Dj. Jovović , Maja M. Ribar (2015).Hospital Pharmacology. 2015; 2(1):220-224 UDC: 615.014.2 Information on the Quality of Substance for the Preparation of Pharmaceutical Drugs in Terms of Hospital Pharmacy
Débora Omena Futuro, Lenise Arneiro Teixeira, et.al (2015).Magistral drugs in hospitalized newborns and children Medicamentos magistrais em recém-nascidos e crianças hospitalizados Agueda Cabral de Souza Pereira, Elaine Silva Miranda, Selma Rodrigues de Castilho, Brazil
Dooms, M., Carvalho, M. (2018). Compounded medication for patients with rare diseases. Orphanet J Rare Dis 13, 1
Jovanović Lješković,Aleksandra Jovanović Galović,Svetlana Stojkov, Nikola Jojić, Slobodan Gigov Umberto Musazzi (2021). Medicine Shortages in Serbia: Pharmacists’ Standpoint and Potential Solutions for a Non-EU Country Nataš, Pharmaceutics. 2021 Apr; 13(4): 448. 2021pharmaceutics13040448
Guendalina Zuccari , Silvana Alfei , Danilo Marimpietri , Valentina Iurilli , Paola Barabino Leonardo Marchitto (2022). Valid Strategy to Combine Efficacy and Safety in Pediatrics Pharmaceuticals (Basel). 17;15(1):108.
Seoane-Viaño , Sarah J. Trenfield , Abdul W. Basit , (2021). Drug Delivery Reviews Translating 3D printed pharmaceuticals: From hype to real-world clinical applications Alvaro Goyanes Advanced Drug Delivery Reviews Volume 174, July 2021, Pages 553-575 Advanced
Giuseppe Manini , Emeric Carlier, JeaDooms, M., Carvalho, M. Compounded medication for patients with rare diseases. Orphanet J Rare Dis 13, 1 (2018). https://doi.org/10.1186/s13023-017-0741-y 04 January 2018 “When there is no on-label or even no off-label treatment for the patients with rare diseases RD pharmacists have to compound the medication.”(12) Pharmaceutics. 2021 Apr; 2021 Mar 26. doi: 10.3390/pharmaceutics13040448 Medicine Shortages in Serbia: Pharmacists’ Standpoint and Potential Solutions for a Non-EU Country Nataša Jovanović Lješković, A. Jovanović Galović, Svetlana Stojkov,Nikola Jojić, and Slobodan Gigov Umberto Musazzi “Backup manufacturing on a small scale (magistral and galenical) could be a good way to overcome some kind of shortages.”(13) Pharmaceuticals (Basel). 2022 Jan , Mini-Tablets: A Valid Strategy to Combine Efficacy and Safety in Pediatrics Guendalina Zuccari , Silvana Alfei , D. Marimpietri , V. Iurilli , Paola Barabino , Leonardo Marchitto DOI: 10.3390/ph15010108 “In the treatment of pediatric diseases PD , mass-produced dosage forms are often not suitable for children. Commercially available medicines CAM are commonly manipulated and mixed with food by caregivers at home, or extemporaneous kinds of medications are routinely compounded in the hospital pharmacies HP to treat the hospitalized children. Despite considerable efforts by regulatory agencies RA, the pediatric population is still exposed to questionable and potentially harmful practices. When designing medicines for children, the ability to fine-tune the dosage while ensuring safety of the ingredients is of paramount and crucial importance. For these kind of scope solid formulations may represent a valid alternative to liquid formulations for their simpler formula and more stability, and, to overcome the problem of swelling ability, mini-tablets could be a practicable option. This review work deals with the different approaches that may be applied to develop mini-tablets intended for pediatrics with a focus on safety of the excipients. Alongside the various conventional method of compression, 3D printing appeared particularly appealing, as it allows to reduce the number of ingredients and to avoid both the mixing of powders and intermediate steps like as granulation. this technique could be well adaptable to the daily galenic preparations of a hospital pharmacy HP, thus leading to a reduction of the common practice of off-label preparations. “(14) Volume 174, July 2021 Advanced Drug Delivery Reviews Translating 3D printed pharmaceuticals: From hype to real-world clinical application Viaño , Sarah J. Trenfield , A. W. Basit , Alvaro Goyanes https://doi.org/10.1016/j.addr.2021.05.003 “Three-dimensional (3D) printing offers the potential to revolutionise the production of pharmaceuticals targeted to the gastrointestinal GI tract by offering a flexible drug product manufacturing platform that can adapt readily to changing market and patient needs . By using digital computer-aided design software to produce medicines in a layer-by-layer manner, 3D printing enables the on-demand production of drug products DP with personalised dosages PD , drug combinations , geometries and release characteristics ; a concept which is currently unattainable and cost inefficient with conventional manufacturing technologies ( tabletting and encapsulation). This technology has been forecast to disrupt a wide range of pharmaceutical applications, ranging from expediting the drug development process DDP and providing benefits for pharmaceutical manufacture, to on demand printing of personalised medicines PM on the front-line and in hard-to-reach areas . “(15) International Journal of Pharmaceutics Volume 569, 5 October 2019 Feasibility study into the potential use of fused-deposition modeling to manufacture 3D-printed enteric capsules in compounding pharmacies Christoph Nober , Giuseppe Manini , Emeric Carlier , Jean-Marie Raquez , S. Benali , Philippe Dubois , Karim Amighi , Jonathan Goole https://doi.org/10.1016/j.ijpharm.2019.118581 “The purpose of this research work was to investigate the feasibility to manufacture enteric capsules, which could be used in compounding pharmacies , by fused-deposition modeling. It is well-known that conventional enteric dip coating of capsules CPS in community pharmacies CP or hospitals is a time-consuming process which is characterized by an erratic efficacy. Fused-deposition FD modeling was selected as a potential 3D printing method due its ease and low-cost implementation LCI . Before starting to print the capsules CPS , an effective sealing system was designed via a computer-aided design program. Hot melt extrusion was used to make printable enteric filaments. They were made of the enteric polymer, a plasticizer and a thermoplastic polymer, namely Eudragit® L100-55, polyethylene glycol 400 and polylactic acid, respectively. Riboflavine-5′-phosphate was selected as a coloured drug model to compare the efficacy of the 3D printed capsules to that of enteric dip coated capsules as they are currently produced in community pharmacies and hospitals HP . Different parameters of fabrication which could influence the dissolution profile of the model drug, such as the layer thickness or post-processing step, were studied. It was demonstrated that our 3D printed enteric capsules did not release the drug for 2 h in acid medium (pH 1.2). They completely dissolved within 45 min at pH 6.8 which allowed the release of a minimal amount of 85% w/w of drug as it was recommended by the European Pharmacopoeia EP 9th Edition for enteric products.”(16an-Marie Raquez , Samira Benali Philippe Dubois , Karim Amighi , Jonathan Goole (2019).International Journal of Pharmaceutics Feasibility study into the potential use of fused-deposition modeling to manufacture 3D-printed enteric capsules in compounding pharmacies Volume 569, International Journal of Pharmaceutics
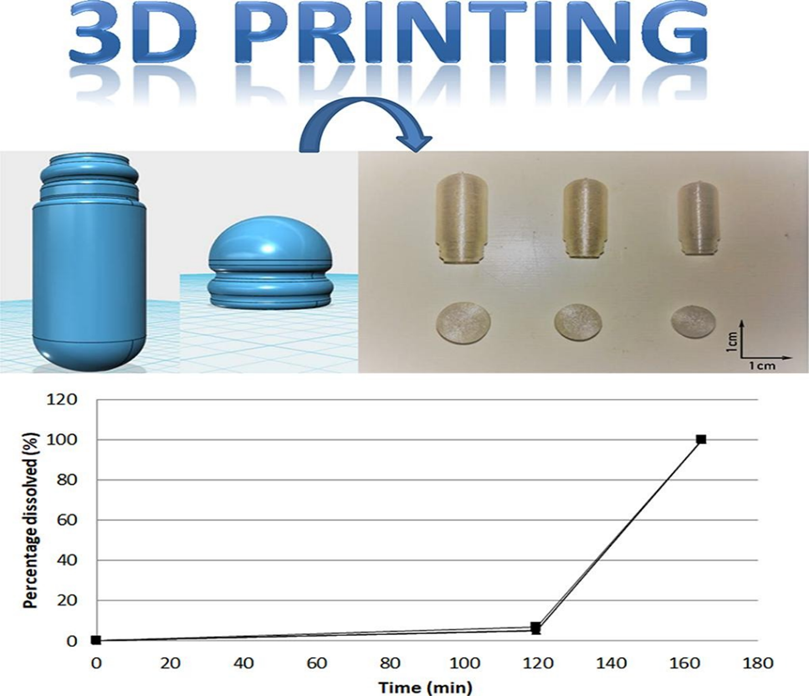
Figure 3: from https://doi.org/10.1016/j.ijpharm.2019.118581
Practical project
In this part are analyzed the italian normative rules named NBP and semplified as DM Salute 18.11.2003 norme di buona preparazione” applied by law as mandatory in the galenic laboratory setting inside the pharmacy ( public or private – hospital and comunity ).
NBP or GMP good manifacturing practice
The GMP philosophy are based on :
documentation of the process , registrations, every phases of the process, activity and single operations.
- team must receive adeguate training
-responsability clearly identified
-Quality of API and eccipients
- cleaning and sanitization procedure
-Regular check of the instruments
- process validation
- NC non conformity management
So In italy by law the pharmacist that work in a galenic laboratory according DM 22/06/05 must to follow or the FULL NBP of ITALIAN FU ( more complex) or DM Salute 18.11.2003 ( if not sterile magistral preparations or officinal reduced scale ) The pharmacy that prepare non sterile magistral formula or officinal reduced scale can follow or full NBP or semplified NBP. Instead if prepared sterile products , or toxic preparates , anticancer drugs and radiodrugs, it must to be used biological hood : it is mandatory to follow full NBP. For non sterile products it is possible in italy to deviate from full NBP and to follow the semplified rules if it is possible to keep under control all the process , prooving it. (quality efficacy, safety depends on organization and consistent control) .First NBP was introduced in (FU IX ed.) in 1989.In the chapter 795 USP, pharmaceutical compounding of non sterile products, related the difficulty of the preparations, its stability , storage conditions, dosage form , complexity in calculations,sistemic, topic use, risk level for pharmacist, damage risk for patients are classified 3 situations :simple compounding , moderate and complex compounding . It is request to produce the master formulation records and the compounding record. Like NBP the USP rules are based on the quality of final products and on the documentation of all process. Some preparations at high microbiological quality need to be prepared in zone with HEPA FILTER . A translation of the meaning of the Semplified NBP an its phylosophy (DM Salute 18.11.2003 ) and their meaning are reported : Application field :( non sterile magistral and officinal forms reduced lots) for hospital and comunity pharmacy medical prescription for magistral formula and Pharmacopeia for Officinal reduced scale production) Preliminar evaluation about opportunity -possibility to prepare the galenic requested or needed
definitions: magistral formula, officinal, reduced batch
laboratory hygienic written procedure, frequency ( provided by director of the pharmacy or lab. Responsible). lab area: it must to be adequate to the kind of galenic products produced, ceiling and walls wahable it can be in a separate room separate or not separate inside the pharmacy.
instruments: mandatory according PHARMACOPEA uff. ITALIAN table n. 6, the measure insntrument must to be verified in regular way. The refrigerattor must to be cleaned. Containers (and related certificate of conformity to pharmacopeia requirement of the primary contaniners).
Raw material : chemical denomination, date of arrive in pharmacy , batch number , expiration date or date of retitulation, certificate of analisys signed by producer ( according pharmacopeia quality requirement), conservation condition or use, date of first use . ( necessary a register of raw materials , eccipients and API, with a progressive numeration). The empty container of raw material must to be keeped for 6 month after final use. Fulfillments ( preventive and after setup ) to the preparations . Prescription verify, normative requirement , sign of the phisician, iperdosages verify ( according table n. 8 pharmacopeia italy), Incompatibility verify , the possibility to prepare in lab. After setup: to be writed on the prescription the progressive number of the preparartion, date of the praparation, expiration date, eccipients used, precautions and cautions, label must to be attached. Sign of the pharmacist in the label , on the prescription or on the working sheet Labeling etichettatura – batch number and expiration date, composition qualitaticve quantitative , API Eccipients , date limits for use, precautions, Price ( comunity pharmacy) Douments storage -conservazione documentazione ( time ) , emplty bottle . The written prescritption must to be kepped in pharmacy for 6 month and the same working sheet. (working sheet). The prescription of narcotics must to be keeped in pharmacy for the time required by normative rules. Quality control: right following of the procedure, organolectic characteristics, control of the pakaging, sealing of the container , righ label compilation, mass uniformity ,acceptation limits A copy of the label must to be attached to the working sheet Documentation : of the working space, instruments, raw materials
xpiration time of the drugs prepared : accordin FU requirement : 30 days that can be prolonged to 6 month acording chemico -phisical microbiological stability documentated by official informations.
Mandatory equipment and tools utensili in pharmacy a ( TABLE N 6 pharmacopea italian in force)
1. balance sensitivity to the mg , scale = 0,001 g, loading capacity at least 500 g or in alternative way two different balances , one with sensitivity at the mg (d=0,001g) with loading capacity at least 50 g and the other with sensitivity at 0,50 g (d=0,50 g) with carry load at least of 2 kg.
2. Bain marie or other equipement that can assure ,in heating , temperature since to 100 °C.
3. Fridge able to assure the right storage conditions according pharmacopeia requirement
4. Point of fusion equipement . ( to test the raw material)
5. chemical glassware, also graduated sufficient for the execution of the preparation.
6. percolator – at empty Concentrator (1).
7. encapsulator (2)
8. Tablet press (3).
9. powder Aspiration system (4).
10. moulds or plastic valve for ovules and suppositories (5).
11.tools and devices necessary to guaranteee sterility of the preparation (6)
Beyond the reported instruments, the pharmacy must have all other instruments,equipements ,tools , materials, products and reactive adequate to the number end to the nature of the preparations usually performed and of suitable tools for their check to be done according the the Pharmacopeia indications.
Pharmacy that execute injectable preparations must have also materials, equipements, and tools essential to this preparations an for all the control expected by pharmacopeia for this specific kind of preparation.
Note:
1)– mandatory for pharmacy that prepare extracts. they must to be of marterials and adequate dimension to the volume and related the preparation to be executed.
(2) - mandatory for the pharmacy that prepare capsules.
(3) – mandatory for the pharmacy that prepare tablets .
(4) – mandatory for pharmacy that prepare tablets, capsules, capsule, teas or sachets .
(5) – mandatory for pharmacy that prepare suppositories and ova .
(6) - for pharmacy that prepare sterile products.
In order to evaluate the application of REDUCED NBP in an hosp. galenic lab PC AREA are reported
The all official non conformity registered from 2008 to 2023. (the internal production)
Results: no major non conformity registered related the preparation activity . (for the non serile galenic activity), only 2 secondary- minor NC due by an excipient to be modified to increase solubility of an API and related a closing system for an oral power .
As reported in this work are clear the advantages to produce some kinds of drugs in a galenic laboratory.
Even the industrial epoca , with the pharmaceutical industry increase , the industrial production of drugs
Was rapidly developed and so reduced or stopped the production in the galenic laboratory :
this process was due to The complexity process to produce with high quality the finished drugs in the amonut requested by the hospital and the patient.
But the same some condition needed to manteign this procedure : for magistral prescription single patient based and for the production of disinfectants, reagents or other product.
It must also to be remembered that during LAST COVID-19 PANDEMIA one of the main producers of antispetic gel hands and alcoolic solution was the hospital pharmacy in their lab as well as in the private pharmacy.
The industry in this situation was not able to provide ready to use great amount of this product in few time as needed for the public safety. (3)
The galenic hospital laboratory in the public hospital was able to guarantee this production and the safety of the patient and healthcare professional.
The results of a practical experience reported show also in a specific settings the goodness of this rules NBP even if semlified .
The technological innovation make possible to better cover the need for drugs shortcomings.
Comparing full NBP to the reduced DM 10nov 2003 it is possible to verify that NBP require separate or separable locals , chek by other pharmacist vs the one that prapare the drug in lab., and required as mandatory written procedures accreditated .
For DM it is not mandatory complex quality check on the final products, no mandatory written procedure are needed (even if suggested).
In this work it is also submitted a new technology useefull in galenica laboratory : the 3D PRINTING SYSTEM
As an innovation for quality and efficiency of the process.
Limitations of the work: the practical experience submiteed is related only to on local centre even if a great hospital that cover the need of about 200.000 citizens ( populations) .
As conclusion it is possible to say that observing the Italian Reduced NBP rules in an advanced countries
can be applied also in non advanced countries with great benefit for healthcare of the patients.
Related the specific preparation requested by the clinicians.
This rules report general behavior and procedure to be followed to be sure that the durgs produced are safe and useful for the patiens.
Not all laboratory in the world have the same intruments or complex laboratory, but in every laboratory
It is crucial to know the responsability as well as procedure adopeted (quality control of raw material,
active substantie, qualification of the pharmacist, traceability of the lots and other.)
For this reason it is opinion of the author that this rules must to be translated in their general meaning from italian to english languages as reported in this work.
The authors submit to the reseachers and pharmacists a new innovative tool: the 3D PRINTING systems for galenic laboratory use : a system to increase global efficiency of the preparation of capsules or other pharmaceutical form during a period of drug shortages.
no
considered all rules
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.
Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.

Clinical Cardiology and Cardiovascular Interventions I testity the covering of the peer review process, support from the editorial office, and quality of the journal.

Clinical Cardiology and Cardiovascular Interventions, we deeply appreciate the interest shown in our work and its publication. It has been a true pleasure to collaborate with you. The peer review process, as well as the support provided by the editorial office, have been exceptional, and the quality of the journal is very high, which was a determining factor in our decision to publish with you.
The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews journal clinically in the future time.

Clinical Cardiology and Cardiovascular Interventions, I would like to express my sincerest gratitude for the trust placed in our team for the publication in your journal. It has been a true pleasure to collaborate with you on this project. I am pleased to inform you that both the peer review process and the attention from the editorial coordination have been excellent. Your team has worked with dedication and professionalism to ensure that your publication meets the highest standards of quality. We are confident that this collaboration will result in mutual success, and we are eager to see the fruits of this shared effort.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, I hope this message finds you well. I want to express my utmost gratitude for your excellent work and for the dedication and speed in the publication process of my article titled "Navigating Innovation: Qualitative Insights on Using Technology for Health Education in Acute Coronary Syndrome Patients." I am very satisfied with the peer review process, the support from the editorial office, and the quality of the journal. I hope we can maintain our scientific relationship in the long term.
Dear Monica Gissare, - Editorial Coordinator of Nutrition and Food Processing. ¨My testimony with you is truly professional, with a positive response regarding the follow-up of the article and its review, you took into account my qualities and the importance of the topic¨.

Dear Dr. Jessica Magne, Editorial Coordinator 0f Clinical Cardiology and Cardiovascular Interventions, The review process for the article “The Handling of Anti-aggregants and Anticoagulants in the Oncologic Heart Patient Submitted to Surgery” was extremely rigorous and detailed. From the initial submission to the final acceptance, the editorial team at the “Journal of Clinical Cardiology and Cardiovascular Interventions” demonstrated a high level of professionalism and dedication. The reviewers provided constructive and detailed feedback, which was essential for improving the quality of our work. Communication was always clear and efficient, ensuring that all our questions were promptly addressed. The quality of the “Journal of Clinical Cardiology and Cardiovascular Interventions” is undeniable. It is a peer-reviewed, open-access publication dedicated exclusively to disseminating high-quality research in the field of clinical cardiology and cardiovascular interventions. The journal's impact factor is currently under evaluation, and it is indexed in reputable databases, which further reinforces its credibility and relevance in the scientific field. I highly recommend this journal to researchers looking for a reputable platform to publish their studies.

Dear Editorial Coordinator of the Journal of Nutrition and Food Processing! "I would like to thank the Journal of Nutrition and Food Processing for including and publishing my article. The peer review process was very quick, movement and precise. The Editorial Board has done an extremely conscientious job with much help, valuable comments and advices. I find the journal very valuable from a professional point of view, thank you very much for allowing me to be part of it and I would like to participate in the future!”

Dealing with The Journal of Neurology and Neurological Surgery was very smooth and comprehensive. The office staff took time to address my needs and the response from editors and the office was prompt and fair. I certainly hope to publish with this journal again.Their professionalism is apparent and more than satisfactory. Susan Weiner

My Testimonial Covering as fellowing: Lin-Show Chin. The peer reviewers process is quick and effective, the supports from editorial office is excellent, the quality of journal is high. I would like to collabroate with Internatioanl journal of Clinical Case Reports and Reviews.

My experience publishing in Psychology and Mental Health Care was exceptional. The peer review process was rigorous and constructive, with reviewers providing valuable insights that helped enhance the quality of our work. The editorial team was highly supportive and responsive, making the submission process smooth and efficient. The journal's commitment to high standards and academic rigor makes it a respected platform for quality research. I am grateful for the opportunity to publish in such a reputable journal.
My experience publishing in International Journal of Clinical Case Reports and Reviews was exceptional. I Come forth to Provide a Testimonial Covering the Peer Review Process and the editorial office for the Professional and Impartial Evaluation of the Manuscript.

I would like to offer my testimony in the support. I have received through the peer review process and support the editorial office where they are to support young authors like me, encourage them to publish their work in your esteemed journals, and globalize and share knowledge globally. I really appreciate your journal, peer review, and editorial office.
Dear Agrippa Hilda- Editorial Coordinator of Journal of Neuroscience and Neurological Surgery, "The peer review process was very quick and of high quality, which can also be seen in the articles in the journal. The collaboration with the editorial office was very good."

I would like to express my sincere gratitude for the support and efficiency provided by the editorial office throughout the publication process of my article, “Delayed Vulvar Metastases from Rectal Carcinoma: A Case Report.” I greatly appreciate the assistance and guidance I received from your team, which made the entire process smooth and efficient. The peer review process was thorough and constructive, contributing to the overall quality of the final article. I am very grateful for the high level of professionalism and commitment shown by the editorial staff, and I look forward to maintaining a long-term collaboration with the International Journal of Clinical Case Reports and Reviews.
To Dear Erin Aust, I would like to express my heartfelt appreciation for the opportunity to have my work published in this esteemed journal. The entire publication process was smooth and well-organized, and I am extremely satisfied with the final result. The Editorial Team demonstrated the utmost professionalism, providing prompt and insightful feedback throughout the review process. Their clear communication and constructive suggestions were invaluable in enhancing my manuscript, and their meticulous attention to detail and dedication to quality are truly commendable. Additionally, the support from the Editorial Office was exceptional. From the initial submission to the final publication, I was guided through every step of the process with great care and professionalism. The team's responsiveness and assistance made the entire experience both easy and stress-free. I am also deeply impressed by the quality and reputation of the journal. It is an honor to have my research featured in such a respected publication, and I am confident that it will make a meaningful contribution to the field.

"I am grateful for the opportunity of contributing to [International Journal of Clinical Case Reports and Reviews] and for the rigorous review process that enhances the quality of research published in your esteemed journal. I sincerely appreciate the time and effort of your team who have dedicatedly helped me in improvising changes and modifying my manuscript. The insightful comments and constructive feedback provided have been invaluable in refining and strengthening my work".

I thank the ‘Journal of Clinical Research and Reports’ for accepting this article for publication. This is a rigorously peer reviewed journal which is on all major global scientific data bases. I note the review process was prompt, thorough and professionally critical. It gave us an insight into a number of important scientific/statistical issues. The review prompted us to review the relevant literature again and look at the limitations of the study. The peer reviewers were open, clear in the instructions and the editorial team was very prompt in their communication. This journal certainly publishes quality research articles. I would recommend the journal for any future publications.

Dear Jessica Magne, with gratitude for the joint work. Fast process of receiving and processing the submitted scientific materials in “Clinical Cardiology and Cardiovascular Interventions”. High level of competence of the editors with clear and correct recommendations and ideas for enriching the article.

We found the peer review process quick and positive in its input. The support from the editorial officer has been very agile, always with the intention of improving the article and taking into account our subsequent corrections.

My article, titled 'No Way Out of the Smartphone Epidemic Without Considering the Insights of Brain Research,' has been republished in the International Journal of Clinical Case Reports and Reviews. The review process was seamless and professional, with the editors being both friendly and supportive. I am deeply grateful for their efforts.
To Dear Erin Aust – Editorial Coordinator of Journal of General Medicine and Clinical Practice! I declare that I am absolutely satisfied with your work carried out with great competence in following the manuscript during the various stages from its receipt, during the revision process to the final acceptance for publication. Thank Prof. Elvira Farina

Dear Jessica, and the super professional team of the ‘Clinical Cardiology and Cardiovascular Interventions’ I am sincerely grateful to the coordinated work of the journal team for the no problem with the submission of my manuscript: “Cardiometabolic Disorders in A Pregnant Woman with Severe Preeclampsia on the Background of Morbid Obesity (Case Report).” The review process by 5 experts was fast, and the comments were professional, which made it more specific and academic, and the process of publication and presentation of the article was excellent. I recommend that my colleagues publish articles in this journal, and I am interested in further scientific cooperation. Sincerely and best wishes, Dr. Oleg Golyanovskiy.

Dear Ashley Rosa, Editorial Coordinator of the journal - Psychology and Mental Health Care. " The process of obtaining publication of my article in the Psychology and Mental Health Journal was positive in all areas. The peer review process resulted in a number of valuable comments, the editorial process was collaborative and timely, and the quality of this journal has been quickly noticed, resulting in alternative journals contacting me to publish with them." Warm regards, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. I appreciate the journal (JCCI) editorial office support, the entire team leads were always ready to help, not only on technical front but also on thorough process. Also, I should thank dear reviewers’ attention to detail and creative approach to teach me and bring new insights by their comments. Surely, more discussions and introduction of other hemodynamic devices would provide better prevention and management of shock states. Your efforts and dedication in presenting educational materials in this journal are commendable. Best wishes from, Farahnaz Fallahian.
Dear Maria Emerson, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. I am delighted to have published our manuscript, "Acute Colonic Pseudo-Obstruction (ACPO): A rare but serious complication following caesarean section." I want to thank the editorial team, especially Maria Emerson, for their prompt review of the manuscript, quick responses to queries, and overall support. Yours sincerely Dr. Victor Olagundoye.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. Many thanks for publishing this manuscript after I lost confidence the editors were most helpful, more than other journals Best wishes from, Susan Anne Smith, PhD. Australian Breastfeeding Association.

Dear Agrippa Hilda, Editorial Coordinator, Journal of Neuroscience and Neurological Surgery. The entire process including article submission, review, revision, and publication was extremely easy. The journal editor was prompt and helpful, and the reviewers contributed to the quality of the paper. Thank you so much! Eric Nussbaum, MD
Dr Hala Al Shaikh This is to acknowledge that the peer review process for the article ’ A Novel Gnrh1 Gene Mutation in Four Omani Male Siblings, Presentation and Management ’ sent to the International Journal of Clinical Case Reports and Reviews was quick and smooth. The editorial office was prompt with easy communication.

Dear Erin Aust, Editorial Coordinator, Journal of General Medicine and Clinical Practice. We are pleased to share our experience with the “Journal of General Medicine and Clinical Practice”, following the successful publication of our article. The peer review process was thorough and constructive, helping to improve the clarity and quality of the manuscript. We are especially thankful to Ms. Erin Aust, the Editorial Coordinator, for her prompt communication and continuous support throughout the process. Her professionalism ensured a smooth and efficient publication experience. The journal upholds high editorial standards, and we highly recommend it to fellow researchers seeking a credible platform for their work. Best wishes By, Dr. Rakhi Mishra.

Dear Jessica Magne, Editorial Coordinator, Clinical Cardiology and Cardiovascular Interventions, Auctores Publishing LLC. The peer review process of the journal of Clinical Cardiology and Cardiovascular Interventions was excellent and fast, as was the support of the editorial office and the quality of the journal. Kind regards Walter F. Riesen Prof. Dr. Dr. h.c. Walter F. Riesen.

Dear Ashley Rosa, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews, Auctores Publishing LLC. Thank you for publishing our article, Exploring Clozapine's Efficacy in Managing Aggression: A Multiple Single-Case Study in Forensic Psychiatry in the international journal of clinical case reports and reviews. We found the peer review process very professional and efficient. The comments were constructive, and the whole process was efficient. On behalf of the co-authors, I would like to thank you for publishing this article. With regards, Dr. Jelle R. Lettinga.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, I would like to express my deep admiration for the exceptional professionalism demonstrated by your journal. I am thoroughly impressed by the speed of the editorial process, the substantive and insightful reviews, and the meticulous preparation of the manuscript for publication. Additionally, I greatly appreciate the courteous and immediate responses from your editorial office to all my inquiries. Best Regards, Dariusz Ziora

Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation, Auctores Publishing LLC, We would like to thank the editorial team for the smooth and high-quality communication leading up to the publication of our article in the Journal of Neurodegeneration and Neurorehabilitation. The reviewers have extensive knowledge in the field, and their relevant questions helped to add value to our publication. Kind regards, Dr. Ravi Shrivastava.

Dear Clarissa Eric, Editorial Coordinator, Journal of Clinical Case Reports and Studies, Auctores Publishing LLC, USA Office: +1-(302)-520-2644. I would like to express my sincere appreciation for the efficient and professional handling of my case report by the ‘Journal of Clinical Case Reports and Studies’. The peer review process was not only fast but also highly constructive—the reviewers’ comments were clear, relevant, and greatly helped me improve the quality and clarity of my manuscript. I also received excellent support from the editorial office throughout the process. Communication was smooth and timely, and I felt well guided at every stage, from submission to publication. The overall quality and rigor of the journal are truly commendable. I am pleased to have published my work with Journal of Clinical Case Reports and Studies, and I look forward to future opportunities for collaboration. Sincerely, Aline Tollet, UCLouvain.

Dear Ms. Mayra Duenas, Editorial Coordinator, International Journal of Clinical Case Reports and Reviews. “The International Journal of Clinical Case Reports and Reviews represented the “ideal house” to share with the research community a first experience with the use of the Simeox device for speech rehabilitation. High scientific reputation and attractive website communication were first determinants for the selection of this Journal, and the following submission process exceeded expectations: fast but highly professional peer review, great support by the editorial office, elegant graphic layout. Exactly what a dynamic research team - also composed by allied professionals - needs!" From, Chiara Beccaluva, PT - Italy.

Dear Maria Emerson, Editorial Coordinator, we have deeply appreciated the professionalism demonstrated by the International Journal of Clinical Case Reports and Reviews. The reviewers have extensive knowledge of our field and have been very efficient and fast in supporting the process. I am really looking forward to further collaboration. Thanks. Best regards, Dr. Claudio Ligresti
Dear Chrystine Mejia, Editorial Coordinator, Journal of Neurodegeneration and Neurorehabilitation. “The peer review process was efficient and constructive, and the editorial office provided excellent communication and support throughout. The journal ensures scientific rigor and high editorial standards, while also offering a smooth and timely publication process. We sincerely appreciate the work of the editorial team in facilitating the dissemination of innovative approaches such as the Bonori Method.” Best regards, Dr. Matteo Bonori.

I recommend without hesitation submitting relevant papers on medical decision making to the International Journal of Clinical Case Reports and Reviews. I am very grateful to the editorial staff. Maria Emerson was a pleasure to communicate with. The time from submission to publication was an extremely short 3 weeks. The editorial staff submitted the paper to three reviewers. Two of the reviewers commented positively on the value of publishing the paper. The editorial staff quickly recognized the third reviewer’s comments as an unjust attempt to reject the paper. I revised the paper as recommended by the first two reviewers.

Dear Maria Emerson, Editorial Coordinator, Journal of Clinical Research and Reports. Thank you for publishing our case report: "Clinical Case of Effective Fetal Stem Cells Treatment in a Patient with Autism Spectrum Disorder" within the "Journal of Clinical Research and Reports" being submitted by the team of EmCell doctors from Kyiv, Ukraine. We much appreciate a professional and transparent peer-review process from Auctores. All research Doctors are so grateful to your Editorial Office and Auctores Publishing support! I amiably wish our article publication maintained a top quality of your International Scientific Journal. My best wishes for a prosperity of the Journal of Clinical Research and Reports. Hope our scientific relationship and cooperation will remain long lasting. Thank you very much indeed. Kind regards, Dr. Andriy Sinelnyk Cell Therapy Center EmCell
