AUCTORES
Globalize your Research
Research Article | DOI: https://doi.org/10.31579/2766-2314/131
1,2 Department of Physiology, Faculty of Medicine, Sabratha University, Libya
3 Department of Biomedical Sciences, School of Basic Sciences, Libyan Academy, Tripoli, Libya
*Corresponding Author: Azab Elsayed Azab, Department of Physiology, Faculty of Medicine, Sabratha University, Libya.
Citation: Azab E. Azab, J. M. Jbireal, and Ruwaydah A. Salem, (2024), Effect of Covid-19 Infection on Haematological and Immune Antibodies Titer among Infected Patients in the Maitega Isolation Centers, Tripoli, Libya, J, Biotechnology and Bioprocessing, 5(1); DOI:10.31579/2766-2314/131
Copyright: © 2024, Azab Elsayed Azab. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 02 January 2024 | Accepted: 12 January 2024 | Published: 19 January 2024
Keywords: coronavirus disease 2019, hematological parameters, immune antibodies titer, d-dimer, crp
Background: Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV- 2) has become a global pandemic causing significant mortality and morbidity.
Objectives: This study aimed to examine the alterations in hematological parameters and immunoglobulin levels in COVID-19 patients and determine any potential correlation between the changes in specific hematological variables and the severity of COVID-19 infection among infected patients in the Maitega Isolation Centers, Tripoli, Libya.
Subjects and Methods: This cross-sectional study was conducted between September 2021 and March 2022. Among 50 infected patients (26 males & 24 females) and 50 healthy individuals (23 males& 27 females) without any chronic disease or respiratory symptoms were recruited for the control group. Structured questionnaires were used to obtain data. 5 ml of venous blood was collected from each participant for estimation of complete blood count (CBC), serum COVID-19 IgM and COVID-19 IgG, Ferritin, C Reactive Protein, and D-dimer using CBC PKL analyzer and The Fluorecare instrument.
Results: The results showed that patients with COVID-19 had a significant (P<0.05) decrease in lymphocytes count and RBCs count at first day of the infection to 7 days, and after 14 days of infection, respectively compared with the healthy individuals, non-statistically significant (P>0.05) changes were observed in hemoglobin concentration, WBCs, granulocytes, and platelets counts compared with the healthy individuals. The patients with COVID-19 had a significant (P<0.0001) increase in IgM levels during 1-7 days of infection compared with healthy individuals, respectively compared with the healthy individuals. Also, IgG levels were showed a gradual significantly (P<0.0001) increase during COVID-19 Virus Infection among COVID-19 patients after 14 days compared with the controls. additionally, coronavirus infection caused a significant (P<0.0001) increase in D-dimer, CRP, and Ferritin levels compared with the healthy control individuals,
Conclusion: It can be concluded that coronavirus infection caused a significant decrease in Lymphocytes count and an increase in IgM, IgG, D-dimer, CRP, and Ferritin levels at different periods compared to the controls. Further studies are needed to confirm these results. COVID-19 specific immunoglobulins and some inflammatory factors in COVID-19 patients. These changes in IgM, IgG, D-dimer, CRP, and Ferritin levels during COVID-19 Virus Infection among COVID-19 patients may help clinicians to better understand COVID-19 and provide more clinical treatment options.
The emergence of the coronavirus disease 2019 (COVID-19) in Wuhan, China marked the beginning of a highly transmissible virus that rapidly spread across the world, leading to a global pandemic (1). This virus, officially known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has posed a significant health challenge due to its high mortality rate and rapid spread (2). As of November 2022, the World Health Organization (WHO) has reported over (637.737.550) confirmed cases of COVID-19 globally, with 6.611.874 recorded deaths (3). Coronaviruses are a family of large viruses within the Corona viridae family (4). These viruses have a single-stranded RNA genome (5) and are surrounded by a helical capsid and a lipoprotein envelope that contains several spicules of glycoprotein, giving the virus a crown-like appearance (6). The SARS-CoV-2 virus can cause severe clinical complications, particularly in elderly patients and those with underlying comorbidities such as diabetes (7), cardio and cerebrovascular diseases (8), obesity, cancer, and pathologies of the digestive, endocrine, nervous, and respiratory systems (9).
SARS-CoV-2, a member of the Coronaviridae family, is a type of coronavirus that has been identified in avian hosts as well as several other species (10). Effective management of COVID-19 infection requires early diagnosis, appropriate treatment, and future control measures to limit the spread of the virus. Result of Laboratory parameters play a crucial role in confirming COVID-19 diagnosis and can help discriminate between severe and non-severe cases, as well as those at high or low risk of mortality (11).
The role of white blood cells, hemoglobin, and platelets in the manifestation of signs and symptoms of coronavirus disease 2019 (COVID-19) has been documented (12). Serological testing, which detects antibodies, is another common laboratory diagnostic tool that can aid in the diagnosis of the disease (13). The detection of IgM and IgG antibodies is particularly useful for serological diagnosis and for understanding the prevalence of the infection in the population, as well as for implementing control measures (14, 15). Antibody testing for SARS-CoV-2 is rapid and sensitive, making it a valuable adjunct for the diagnosis of COVID-19 (15).
During the early stages of (COVID-19) 2019, inflammatory biomarkers such as C-reactive protein (CRP) and Ferritin are notably elevated. Therefore, it is important to screen for inflammation-associated biomarkers and coagulation tests including Ferritin, C-reactive protein, and D-dimer to help in the diagnosis of the disease (16). Recent clinical studies have suggested that CRP and other factors like Ferritin, Coagulation Factors and Inflammatory indexes may be associated with the severity of COVID-19 (17). Many studies found that C-reactive protein is a reliable diagnostic tool for predicting the severity of coronavirus disease 2019 in its early stages. In summary, the available literature suggests that CRP levels could be an indicator of disease severity during the early stage of COVID-19 (18).
This study aimed to examine the alterations in hematological parameters and immunoglobulin levels in COVID-19 patients and determine any potential correlation between the changes in specific hematological variables and the severity of COVID-19 infection among infected patients in the Maitega Isolation Centers, Tripoli, Libya
3.1 Study Type and Design
This study utilized a descriptive cross-sectional observational design.
3.2. Study Population:
The study population included both male and female COVID-19 infected patients from different age groups. Healthy individuals were also included as a control group for comparison purposes.
3.3. Sample Size:
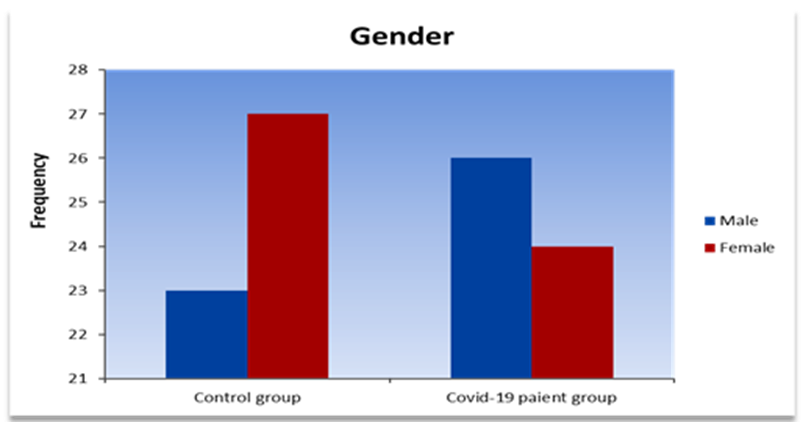
In this study, a total of 150 blood samples were collected from individuals infected with COVID-19, with 100 samples representing cases in the study group. Among the study group, 50 samples were collected within the first 7 days of infection, while another 50 were collected after 14 days. Furthermore, a control group consisting of 50 healthy individuals matched for age and gender was included for comparison purposes. Out of the 50 COVID-19 patients, 26 (52%) were male and 24 (48%) were female, while the control group consisted of 23 (46%) males and 27 (54%) females (Table. 1).
Groups Gender | Control group | Covid-19 patients | ||
| Frequency | Percent (%) | Frequency | Percent (%) | |
| Males | 23 | 46% | 26 | 52% |
| Females | 27 | 54% | 24 | 48% |
Table. 1: Distribution of gender among control group and Covid -19 patients
3.4. Ethical Considerations
This study was conducted with ethical approval from the ethical committee of the Libyan Academy of Science and the Maitega Isolation Centers, which was used as a point for sample collection. Informed consent was obtained from all participants and their families before they were included in the study, ensuring compliance with ethical standards.
3.5. Sample Collection:
Each participant provided a 5 ml venous blood sample in an EDTA tube for complete blood count (CBC) analysis. An additional 3 ml of blood was collected in a plain tube for measurement of ferritin, D-dimer, C-reactive protein (CRP), and Covid-19 IgM, and IgG.
3.6. Hematological Study:
The blood samples for CBC were analyzed using an automated blood analyzer (PKL), following the manufacturer's instructions. Further investigations were conducted for ferritin, D-dimer, CRP, and Covid-19, IgM & Covid-19IgG using manual kit and automatic measurement methods by Flurocare.
3.7. Statistical Analysis:
The normal continuous variables were presented by means and standard errors (SE), the non-normal continuous variables were presented by medians and interquartile range (IQR), categorical variables were presented as counts and percentages. The statistical tools used for analyzing the data is SPSS 27 and Graph Pad Prism 8. The Shapiro-Wilk test is used to assess the normality of the distribution of the continuous variables. The statistical significance of the difference between groups were evaluated by t-test and ANOVA for normal variables, whereas for non-normal continuous variables; Kruskall-Wallis H test is used for comparing more than two independent samples, Mann-Whitney U test is used for comparing two independent samples, and Willcoxon signed rank test is used for comparing two related samples. Chi-square statistical analysis was performed to determine significant values. Pearson correlation coefficient is used to evaluate the relations between continuous variables and spearman rank correlation is used to evaluate the relations between categorical variables. A P-value of less than 0.05 is considered statistically significant.
This study included 150 blood sample from patients infected with COVID-19, 100 of them represented cases (study group): 50 during the first 7 day of infection ,50 repeated test after 14 day, and (50) represented healthy age and gender matched subjects were included as compare group (control group). Out 50 patients with COVID-19, 26 (52%) males and 24(48%) females, were the (control group), 23 (46%) males and 27(54%) females (Figure.1).
The mean ages of the patients was 30% years (40-50) years; while the control group mean age was 24%(60-70) years (Table.2 & Figure.2).
Groups Age Groups | Control group (n=50 | COVID-19 patients’ group (n=50) | ||
| Frequency | Percent (%) | Frequency | Percent (%) | |
| 30 - 40 | 10 | 20 | 1 | 2 |
| 40 - 50 | 9 | 18 | 15 | 30 |
| 50 - 60 | 9 | 18 | 7 | 14 |
| 60 - 70 | 12 | 24 | 14 | 28 |
| 70 - 80 | 5 | 10 | 6 | 12 |
| 80 - 90 | 5 | 10 | 6 | 12 |
| > 90 | 0 | 0 | 1 | 2 |
Table.2: Distribution of age among control group and Covid-19 patients.
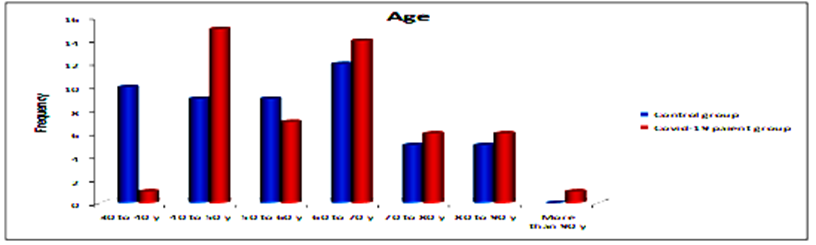
Figure.2: Descriptive of control group and patients according to ages
Table.3 and Figure.3 Shows that (60%) of control group were vaccinated with two doses while (48%) of the patients were, (20%) of control group were vaccinated with one dose while (18%) of the patients were, and (20%) of control group were not vaccinated while (34%) of the patients were non vaccinated.
| Groups | Control group | COVID-19 patients | ||
| Vaccinations | Frequency | (%) | Frequency | ( (% |
| unvaccinated | 10 | 20% | 17 | 34% |
| single dose | 10 | 20% | 8 | 16% |
| 2 doses | 30 | 60% | 25 | 50% |
| Total | 50 | 100% | 50 | 100% |
Table 3: Disripution of vaccinated items of control group and patients
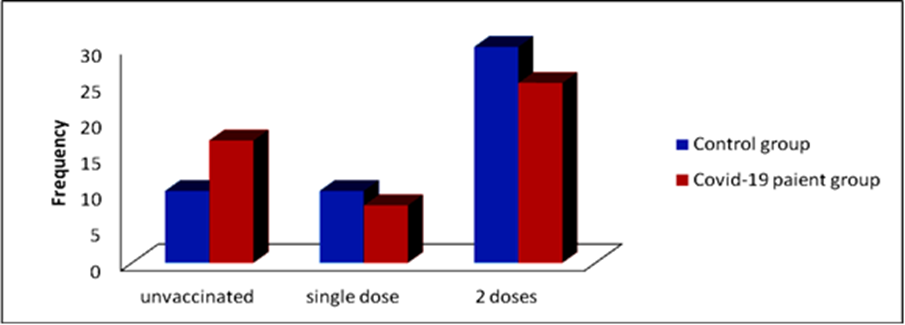
Figure 3: Distribution of control group and patients according to vaccination
Table.4 shows that the IgG means for control group and the patients after 7 days and then after 14 days 0.4004±0.08 and the IgG means for control group and the patients after 7 days and then after 14 days are 0.862±0.32 and 21.49±4.05, respectively (Figures. 4-6).
| P Value | F | 14 days | 7days | Control | Groups Parameters |
| Mean±SE | Mean±SE | Mean±SE | |||
| <0> | 22.07 | 0.032±0.01 | 12.50±2.68 | 0.00±0.00 | Serum IgM (AU/mL) |
| <0> | 26.10 | 21.49±4.05 | 0.862±0.32 | 0.4004±0.08 | Serum IgG (AU/mL) |
Table.4: Serum IgM and IgG levels in control and at 7 and 14 days of COVID-19 Virus Infection
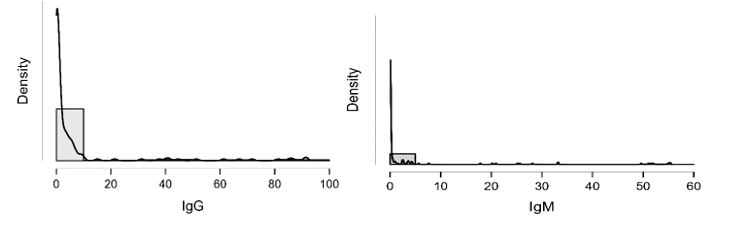
Figure 4: shows the densities of IgG and IgM which is clearly not normally distributed
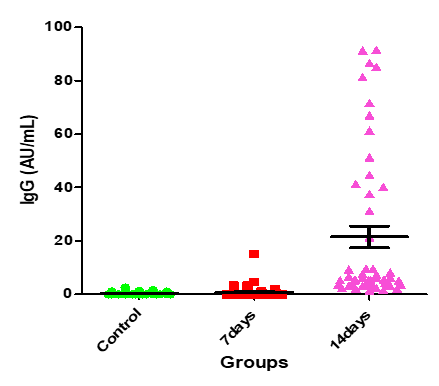
Figure 5: Medians (IQR) of IgG count in control group and the patients group during covid-19 virus infection
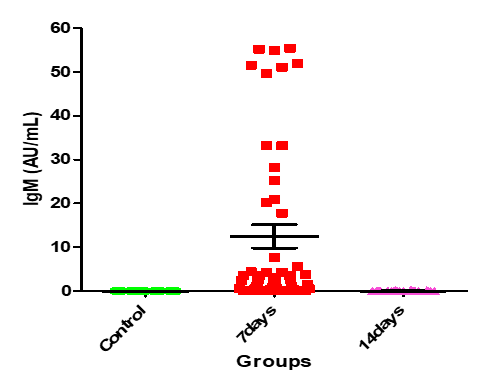
Figure 6: Medians (IQR) of IgM count in control group and the patients group during COVID-19 virus infection
In Table.5, Kolmogorov-Smirnov Z test shows that the IgG of the two independent groups (control group, patients through the first 7 days group) are similar in the shape (have the same distribution) since p-value > α, consequently; Mann-Whitney U test shows that the distributions of the two groups are equal and then there is no significant difference between the medians of the IgG of the two groups. Again, Kolmogorov-Smirnov Z test shows that the IgG of the two independent groups (control group, patients after 14 days group) differs in shape since p-value < α, and then according to Mann-Whitney U test the mean ranks of the two groups differ significantly. Similarly, for the IgM; Kolmogorov-Smirnov Z test shows that the IgM of the two independent groups (control group, patients through the first 7 days group) and again of the two independent groups (control and patients after 14 days) differs in shapes since p-values < α, and then according to Mann-Whitney U test the there is a significant difference between mean ranks of each pair of groups.
| Control | |||||
Group
| Kolmogorov-Smirnov Z | Mann-Whitney U | |||
| parameters | Statistic | p-value | Statistic | p-value | |
| IgG | 0-7 days | 1.000 | 0.270 | 1125.500 | 0.391 |
| 14 days | 4.800 | <0> | 8.000 | <0> | |
| IgM | 0-7 days | 4.700 | <0> | 75.000 | <0> |
| 14 days | 2.900 | <0> | 525.000 | <0> | |
Table 5: Mann-Whitney test for the significance of the differences in ranks of IgG and IgM between controls and patients
From Wilcoxon signed rank test for the paired samples (patients through the first 7 days and patients after 14 days), it is clear that there are statistically significant differences between the ranks of the two groups for IgG and IgM (Table.6)
| N | Mean Rank | Wilcoxon Z | p-value | ||
| IgG14 – IgG7 | Negative Ranks | 3 | 15.00 | -5.720 | <0> |
| Positive Ranks | 47 | 26.17 | |||
| Ties | 0 | ||||
| IgM14 - IgM7 | Negative Ranks | 46 | 24.35 | -5.884 | <0> |
| Positive Ranks | 1 | 8.00 | |||
| Ties | 3 | ||||
Table.6:Wilcoxon signed rank test for the significancy of differences in ranks of IgG and IgM of the patients group during covid-19 virus infection
The IgG mean for non-vaccinated is (9.888±3.477) with median (0.568) and IQR (5.4515), and the IgG mean for one-dose vaccinated is (7.975±3.499) with median (0.861) and IQR (5.2525), whereas the IgG mean for two-dose
vaccinated is (6.143±1.933) with median (0.886) and IQR (3.1548). And the IgM mean for non-vaccinated is (5.376±2.154) with median (0.0135) and IQR (0.522), and for one-dose vaccination is (4.059±2.133) with median (0.0095) and IQR (2.082) (Table.7).
| Mean | Std. Error | Std. Deviation | Min | Max | Median | IQR | ||
| IgG | Not | 9.888 | 3.477 | 23.063 | 0.000 | 91.524 | 0.568 | 5.4515 |
| 1-dosage | 7.975 | 3.499 | 18.516 | 0.020 | 41.325 | 0.861 | 5.2525 | |
| 2-dosages | 6.143 | 1.933 | 17.072 | 0.000 | 86.654 | 0.886 | 3.1548 | |
| IgM | Not | 5.376 | 2.154 | 14.287 | 0.000 | 54.910 | 0.0135 | 0.5220 |
| 1-dosage | 4.059 | 2.133 | 11.285 | 0.000 | 55.210 | 0.0095 | 2.0823 | |
| 2-dosages | 3.710 | 1.306 | 11.530 | 0.000 | 55.400 | 0.000 | 0.1398 |
Table.7: Descriptives of IgG and IgM according to number. of dosages vaccination
Table.8 shows that there are no significant differences In IgG and IgM according to the levels of vaccination and vaccination has a weak effect upon IgG and IgM. For the non-vaccinated individuals, as like as 1-dose vaccinated and 2-dose vaccinated individuals; Kolmogorov-Smirnov Z test shows that the IgG of the two independent groups (control group, patients through the first 7 days group) .there is no significant difference between the distributions of the two groups) since p-value greater than α, consequently; Mann-Whitney U test shows that the distributions of the two groups are equal and
then there is no significant difference between the medians of the IgG of the two groups. But for the two independent groups (control group and the patients after 14 days group) are differ in shape (there is significant difference between the distributions of the two groups) for non-vaccinated, 1-dose vaccinated and 2-dose vaccinated individuals since p-values less than α, consequently; Mann-Whitney U test shows that the distributions of the two groups are not equal; i.e., there is a significant difference between the two groups in mean ranks since p-values less than α.
| Vaccinated | N | Mean Rank | Kruskal-Wallis H | p-value | |
| IgG | NonVaccinated | 44 | 75.92 | 0.302 | 0.860 |
| 1_Dos | 28 | 79.16 | |||
| 2_Dos | 78 | 73.95 | |||
| IgM | Non | 44 | 78.17 | 0.644 | 0.725 |
| 1_Dos | 28 | 78.45 | |||
| 2_Dos | 78 | 72.94 |
Table 8: Kruskal-Wallis signed rank test for the significancy of differences in ranks of IgG and IgM of the patients group during covid-19 virus infection
For the non-vaccinated individuals, as like as 1-dose vaccinated and 2-dose vaccinated individuals; Kolmogorov-Smirnov Z test shows that the IgG of the two independent groups (control group, patients through the first 7 days group) .there is no significant difference between the distributions of the two groups) since p-value greater than α, consequently; Mann-Whitney U test shows that the distributions of the two groups are equal and then there is no significant difference between the medians of the IgG of the two groups. But for the two independent groups (control group and the patients after 14 days group) are differ in shape (there is significant difference between the distributions of the two groups) for non-vaccinated, 1-dose vaccinated and 2-dose vaccinated individuals since p-values less thanα, consequently; Mann-Whitney U test shows that the distributions of the two groups are not equal; i.e., there is a significant difference between the two groups in mean ranks since p-values less than α (Table.9).
| Group | N | Mean Rank | Kolmogorov-Smirnov Z | p-value | Mann-Whitney U | p-value | ||
IgG
| Non vaccinated | Control | 10 | 13.40 | 0.768 | 0.598 | 79.000 | 0.763 |
| 0-7 days | 17 | 14.35 | ||||||
| Control | 10 | 5.50 | 2.509 | <0> | 0.000 | <0> | ||
| 14 days | 17 | 19.00 | ||||||
| 1-dose vaccinated | Control | 10 | 9.20 | 0.846 | 0.471 | 37.000 | 0.513 | |
| 0-7 days | 17 | 10.89 | ||||||
| Control | 10 | 5.50 | 2.176 | <0> | 0.000 | <0> | ||
| 14 days | 17 | 15.00 | ||||||
| 2-dose vaccinated | Control | 30 | 26.38 | 0.700 | 0.711 | 326.500 | 0.560 | |
| 0-7 days | 24 | 28.90 | ||||||
| Control | 30 | 15.67 | 3.408 | <0> | 5.000 | <0> |
Table 9: The significancy of differences between control group and patients group in IgG according to vaccination levels
From Wilcoxon Z test for related samples there is a significant difference between the level of IgG of the patients through the first 7 days and the level of IgG of the patients after 14 days in favor of the last for each of the levels of vaccination, since p-values < α as it shown in Table .10
| IgG14 – IgG7 | N | Mean Rank | Wilcoxon Z | p-value | |
| Not vaccinated | Negative Ranks | 1 | 12.00 | -3.053 | 0.002 |
| Positive Ranks | 16 | 8.81 | |||
| Ties | 0 | ||||
| 1-dose vaccinated | Negative Ranks | 0 | 0.00 | -2.666 | 0.008 |
| Positive Ranks | 9 | 5.00 | |||
| Ties | 0 | ||||
| 2-dose vaccinated | Negative Ranks | 2 | 3.50 | -4.086 | <0> |
| Positive Ranks | 22 | 13.32 | |||
| Ties | 0 | ||||
Table 10: The significancy of differences between patients group through the first 7 days and after 14 days in IgG according to vaccination levels
At table .11 Kolmogorov-Smirnov Z test shows that there is a difference between the distributions of the two independent groups (control and patients through the first 7 days) for the three levels of vaccination since p-values < α, and then by Mann-Whitney U test there is a significant difference between the mean ranks of the two groups since p-values < α. Whereas for the two independent groups (control and patients after 14 days) Kolmogorov-Smirnov Z test shows that there is no difference between the distributions of the two groups for the non-vaccinated and 1-dose vaccinated individuals and then by Mann-Whitney U test there is a significant difference between the
medians since p-value < α, but for the 2-dose vaccinated individuals there is a significant difference between the distributions of the two groups by Kolmogorov-Smirnov Z test since p-value < α, and by Mann-Whitney U test there is a significant difference between mean ranks of the two groups.
Similarly, there is a significant difference between the level of IgM of the patients through the first 7 days and the level of IgM of the patients after 14 days in favor of the first for each of the levels of vaccination, since p-values < α as it shown in table.11.
| Group | N | Mean Rank | Kolmogorov Smirnov Z |
p-value | Mann-Whitney U | p-value | ||
| IgM | Non vaccinated | control | 10 | 6.50 | 2.214 | <0> | 10.000 | <0> |
| 0-7 days | 9 | 18.41 | ||||||
| control | 10 | 9.50 | 1.328 | 0.059 | 40.000 | 0.007 | ||
| 14 days | 9 | 16.65 | ||||||
| 1-dose vaccinated | control | 10 | 5.50 | 2.176 | <0> | 0.000 | <0> | |
| 0-7 days | 9 | 15.00 | ||||||
| control | 10 | 7.00 | 1.451 | 0.030 | 15.000 | 0.003 | ||
| 14 days | 9 | 13.33 | ||||||
| 2-dose vaccinated | control | 30 | 16.00 | 3.499 | <0> | 15.000 | <0> | |
| 0-7 days | 24 | 41.88 | ||||||
| control | 30 | 20.50 | 2.130 | <0> | 150.000 | <0> | ||
| 14 days | 24 | 36.25 |
Table 11: The significance of differences between controls and patients in IgM according to vaccination levels
Shapiro-Wilk test table.12 shows that RBC and Granulocytes are normally distributed such that p-value > 0.05, and the others are not distributed normally since p-value < α. It can be seen clearly in figure.7 that the probability densities of RBC and GRAN are near to norma while the others are much differ of the normal distribution.
| Parameters | WBC | RBC | HGB | PLT | Lymphocytes | Granulocytes | D-dimer | CRP | FerrItin |
|---|---|---|---|---|---|---|---|---|---|
| Test | |||||||||
| Shapiro-Wilk | 0.676 | 0.984 | 0.929 | 0.955 | 0.375 | 0.990 | 0.589 | 0.498 | 0.424 |
| P-value | < 0.001 | 0.075 | < 0.001 | < 0.001 | < 0.001 | 0.338 | < 0.001 | < 0.001 | < 0.001 |
Table12: Shapiro-Wilk test for normality of the distributions of the parameter
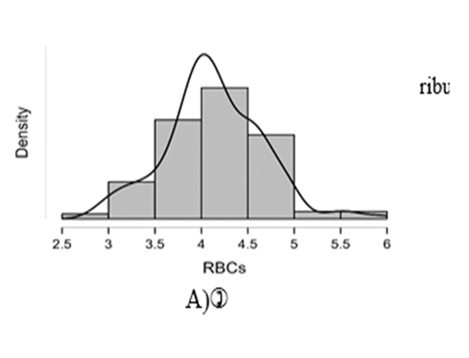
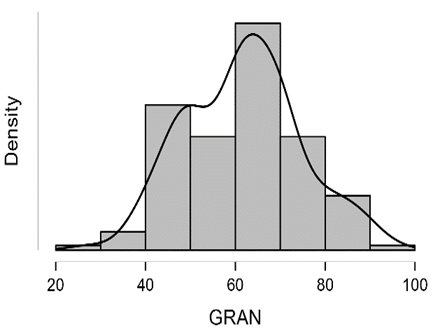
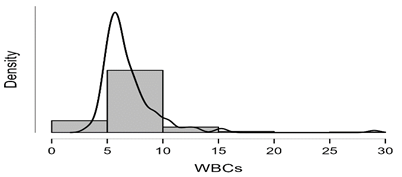
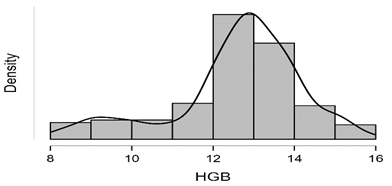
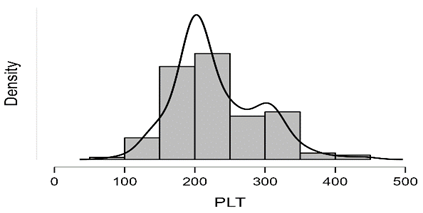
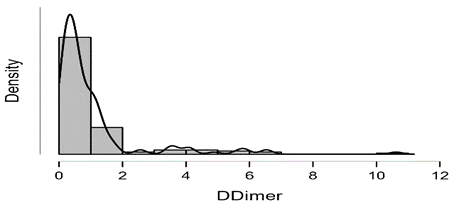
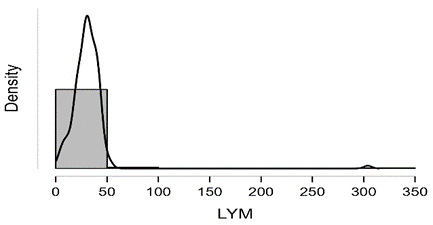
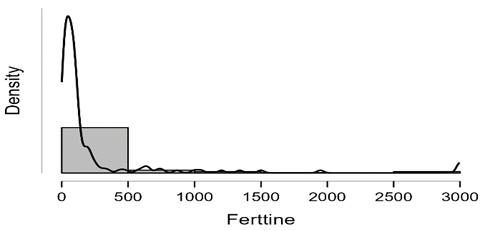
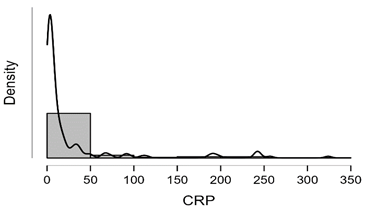
Figure7: Desities of parameters distributions
t-test for independent samples table.13 and figure 8-13 show that there are significant differences between the means of RBC for control and patients through first 7 days, as such as for controls and patients after 14 days such that p-values < α. While from t-test for paired samples there is no significant difference between means of RBC for the patients through the first 7 days and after 14 days as p-value > α. The last figures showed that t test for the paired samples (patients through the first 7 days and patients after 14 days), it is clear that there are no statistically significant differences between the ranks of the two groups for WBC, HGB, PLT and Lymphocytes since p-values > α.
| P Value | F | 14 days of infection | 7days of infection | Control | Groups Parameters |
| Mean±SE | Mean±SE | Mean±SE | |||
| 0.0014 | 6.899 | 4.21±0.07 | 4.27±0.07 | 3.92±0.08 | RBCs count (x 106/μL) |
| 0.1408 | 1.987 | 12.44±0.24 | 12.38±0.27 | 12.95±0.14 | Hemoglobin (g/dl) |
| 0.1848 | 1.708 | 6.47±0.21 | 7.15±0.42 | 6.52±0.20 | WBCs count (x 103/μL) |
| 0.0017 | 6.671 | 29.19±1.08 | 26.34±1.83 | 33.46±1.13 | Lymphocytes % |
| 0.094 | 2.403 | 63.98±1.36 | 62.29±2.29 | 58.52±1.63 | Granulocytes % |
| 0.8495 | 0.1633 | 232.00±11.24 | 232.00±11.24 | 224.90±7.00 | Platelets Count (x103/µL) |
Table 13: Variations in hematological parameters
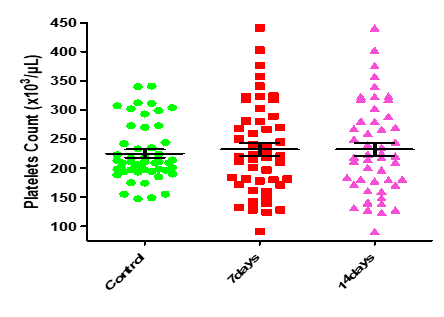
Figure8: Means (std.dev.) of RBC count in control group and the patients group during covid-19 virus infection
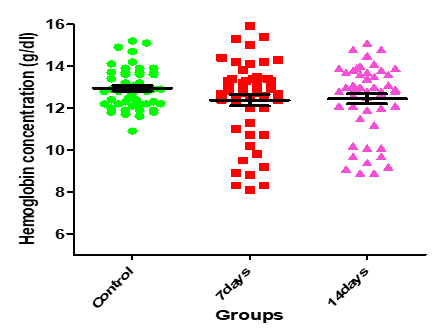
Figure 9: Medians (IQR) of HGB count in control group and the patients group during COVID-19 virus infection
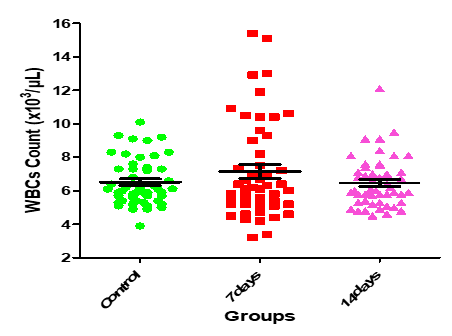
Figure10: Medians (IQR) of WBC count in control group and the patients group during covid-19 virus infection
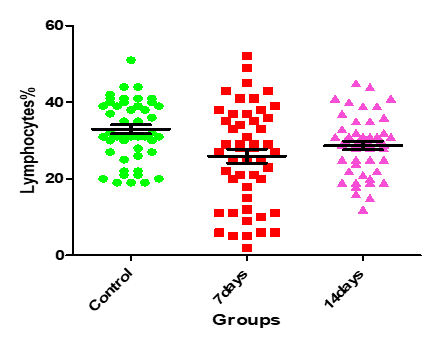
Figure11: Medians (IQR) of Lymphocgtes count in control group and the patients group during COVID-19 virus infection
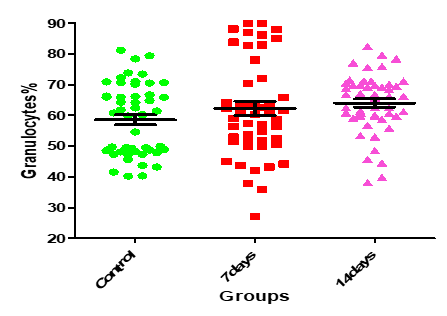
Figure12: Means (std.dev.) of Granulocytes count in control group and the patients group during covid-19 virus infection
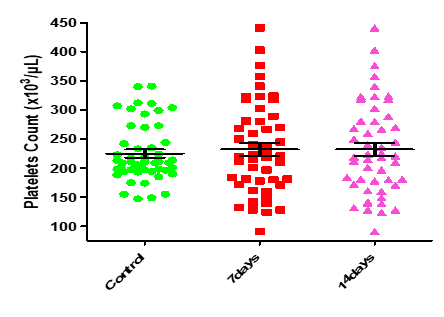
Figure 13: Medians (IQR) of PLT count in control group and the patients group during covid-19 virus infection
Table.14 shows that the mean of RBC for the non-vaccinated individuals is 4.255 with std. deviation OF 0.610, while the mean value of RBC after the 1-dose vaccinated individuals is 4.229 with std. deviation 0.515, whereas the mean value of RBC after the 2-dose vaccinated individuals is 4.033 with std. deviation 0.470.
| RBC | N | Mean | Std. Deviation | Std. Error | Minimum | Maximum | |
| Non | 44 | 4.255 | 0.610 | 0.0919 | 2.860 | 5.930 | |
| 1_Dos | 28 | 4.229 | 0.515 | 0.0973 | 3.210 | 5.380 | |
| 2_Dos | 78 | 4.033 | 0.470 | 0.0532 | 2.970 | 5.010 | |
| Total | 150 | 4.135 | 0.530 | 0.0433 | 2.860 | 5.930 |
Table 14: Descriptive of RBC according to vaccination levels
ANOVA test.15 shows that there is no any significant difference between the means of RBC according to vaccination levels as p-value < α, and the vaccination has a weak effect upon RBC since η2=0.04 less than 0.06
| Sum of Squares | df | Mean Square | F | p-value | |
| Between Groups | 1.690 | 2 | 0.845 | 3.095 | 0.048 |
| Within Groups | 40.139 | 147 | 0.273 | ||
| Total | 41.829 | 149 |
Table 15: ANOVA test for the significance of differences between the means of RBC according to the vaccination levels
Table .16 shows that the mean of Granulocyte for the non-vaccinated individuals is 60.90 with std. deviation 13.98, while the mean of Granulocytes for the 1-dose vaccinated individuals is 58.63 with std. deviation 12.50, whereas the mean of GRAN for the 2-dose vaccinated individuals is 63.05 with std. deviation 12.30.
| N | Mean | Std. Deviation | Std. Error | Min | Max | ||
| Non-Vaccinated | 44 | 60.8955 | 13.97762 | 2.10721 | 27.00 | 89.90 | |
| 1_Dos | 28 | 58.6250 | 12.50471 | 2.36317 | 39.80 | 89.90 | |
| 2_Dos | 78 | 63.0535 | 12.29573 | 1.39222 | 40.20 | 94.70 | |
| Total | 150 | 61.5938 | 12.87490 | 1.05123 | 27.00 | 94.70 |
Table 16: Descriptives of Granulocytes according to vaccination levels
From table.17 we can easily observe that for the non-vaccinated individuals the mean of WBC is 6.759 with std. deviation 2.332 and the median is 6.1, for HGB the mean is 12.322 with std. deviation 2.198 and median is 12.95, the PLT mean is 219.55 with std. deviation 57.368 and median is 207.5 and the LYM mean is 20.269 with std. deviation 10.478 and median is 31.5. For the 1-dose vaccinated individuals the WBC mean is 6.096 with std. deviation 1.55 and median is 5.8, the HGB mean is 12.841 with std. deviation 1.117
and median is 12.8, the PLT mean is 243.07 with std. deviation 63.822 and median is 223 and the LYM mean is 32.504 with std. deviation 10.004 and median is 31.6. finally for the 2-dose vaccinated individuals the WBC mean is 7.154 with std. deviation 3.224 and median is 6.2, the HGB mean is 12.65 with std. deviation 1.301 and median is 12.8, the PLT mean is 231.78 with std. deviation 61.072 and median is 211 and the LYM mean is 31.827 with std. deviation 32.572 and median is 28.6.
| Vaccinated | Mean | Std. Deviation | Min | Max | median50% | IQR | |
| WBC | Non | 6.759 | 2.332 | 3.40 | 15.40 | 6.10 | 1.88 |
| 1_Dose | 6.096 | 1.550 | 3.20 | 9.60 | 5.80 | 1.70 | |
| 2_Dose | 7.154 | 3.224 | 4.40 | 29.00 | 6.20 | 2.70 | |
| HGB | Non | 12.322 | 2.198 | 8.10 | 15.90 | 12.95 | 3.57 |
| 1_Dose | 12.841 | 1.117 | 10.20 | 15.10 | 12.80 | 1.60 | |
| 2_Dose | 12.650 | 1.301 | 8.90 | 15.40 | 12.80 | 1.30 | |
| PLT | Non | 219.55 | 57.368 | 91 | 358 | 207.50 | 78 |
| 1_Dose | 243.07 | 63.822 | 132 | 376 | 223.00 | 103 | |
| 2_Dose | 231.78 | 61.072 | 128 | 441 | 211.00 | 74 | |
| LYM | Non | 30.269 | 10.478 | 5.40 | 49.90 | 31.50 | 12.90 |
| 1_Dose | 32.504 | 10.004 | 5.00 | 52.90 | 31.60 | 10.80 | |
| 2_Dose | 31.827 | 32.572 | 2.90 | 304.00 | 28.60 | 15.41 |
Table 17: Descriptives of WBC, HGB, PLT and Lymphocytes according to vaccination levels
Table .18 shows that there are no significant differences in WBC, HGB, PLT and Lymphocytes according to the levels of vaccination and vaccination has a weak effect upon WBC, HGB, PLT and Lymphocytes since ε2 less than 0.04.
| Vaccinated | N | Mean Rank | Kruskal-Wallis H | p-value | |
| WBC | Non-Vaccinated | 44 | 75.22 | 3.494 | 0.174 |
| 1_Dos | 28 | 62.46 | |||
| 2_Dos | 78 | 80.34 | |||
| HGB | Non | 44 | 75.31 | 0.057 | 0.972 |
| 1_Dos | 28 | 77.25 | |||
| 2_Dos | 78 | 74.98 | |||
| PLT | Non | 44 | 69.09 | 2.270 | 0.322 |
| 1_Dos | 28 | 84.89 | |||
| 2_Dos | 78 | 75.74 | |||
| LYM | Non | 44 | 78.61 | 4.459 | 0.108 |
| 1_Dos | 28 | 88.54 | |||
| 2_Dos | 78 | 69.06 |
Table 18: The significance of differences in WBC, HGB, PLT and Lymphocytes according to the levels of vaccination
Table.19 ad figures 14-16 show that the distributions of D-dimer for the two groups are differ in shape by t test Z test since p-values < controls> α, there is no significant difference between the distributions of the groups controls and the patients after 14 days as p-values < α, i.e. there is no significant difference between the controls and the patients after 14 days Ferritin level.
| P value | F | 14 days of inf. | 7days of inf. | Control | Groups Parameters |
| Mean±SE | Mean±SE | Mean±SE | |||
| <0> | 10.10 | 1.221±0.21 | 1.584±0.28 | 0.333±0.04 | Serum D-dimer (µg/ml) |
| <0> | 11.82 | 24.89±6.33 | 57.03±12.05 | 3.31±0.30 | Serum C-Reactive Protein (mg/L) |
| 0.0008 | 7.451 | 203.8±49.28 | 457.8±117.4 | 61.02±7.18 | Serum Ferritin (µg/L) |
Table 19: Serum D-dimer, C- reactive protein (CRP), and Ferritin levels in control and at 7 and 14 days of COVID-19 Virus Infection.
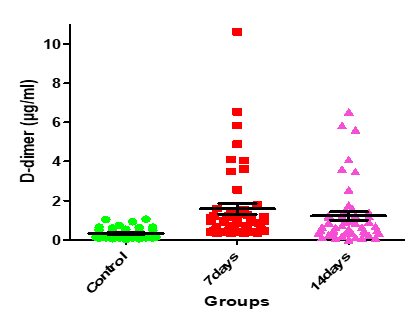
Figure 14: Medians (IQR) of D-dimer count in control group and the patients group during COVID-19 virus infection
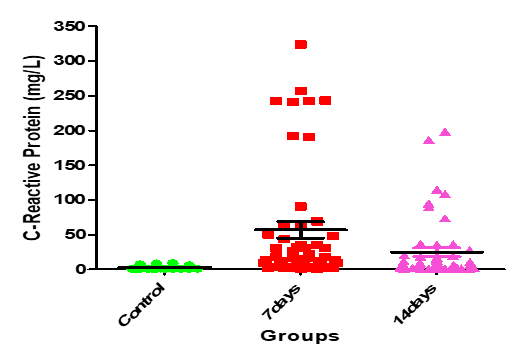
Figure 15: Medians (IQR) of CRP count in control group and the patients group during COVID-19 virus infection
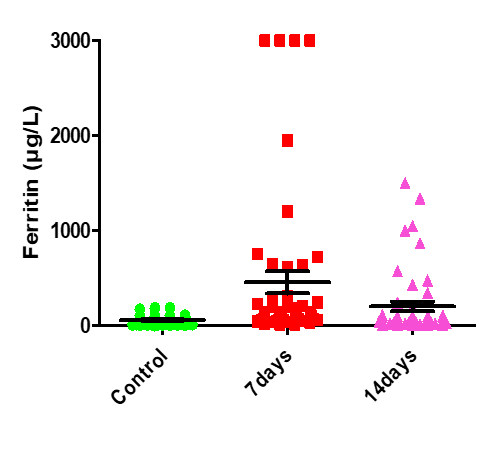
Figure 16: Medians (IQR) of Ferritin count in control group and the patients group during COVID-19 virus infection
Table.20 shows that, for the non-vaccinated individuals the mean of D-dimer is 1.456 with std. deviation 1.698 and the median is 0.638, for CRP the mean is 36.001 with std. deviation 67.75 and median is 5.255, the Ferritin mean is 351.141 with std. deviation 702.675 and median is 93.595. For the 1-dose vaccinated individuals the D-dimer mean is 0.530 with std. deviation 0.333
and median is 0.450, the CRP mean is 13.556 with std. deviation 20.869 and median is 5.020, the Ferritin mean is 102.837 with std. deviation 151.601 and median is 67.335. Finally for the 2-dose vaccinated individuals the D-dimer mean is 1.000 with std. deviation 1.616 and median is 0.591, the CRP mean is 29.460 with std. deviation 63.401 and median is 5.985, the Ferritin mean is 226.616 with std. deviation 518.658 and median is 78.450.
| Parameters | Vaccinated | Mean | Std. Deviation | Min | Max | 50% | IQR | |
| D-dimer | Non-Vaccinated | 1.456 | 1.698 | 0.012 | 6.531 | 0.638 | 1.951 | |
| 1_Dos | 0.530 | 0.333 | 0.021 | 1.212 | 0.450 | 0.379 | ||
| 2_Dos | 1.000 | 1.616 | 0.053 | 10.620 | 0.591 | 0.889 | ||
| CRP | Non-Vaccinated | 36.001 | 67.750 | 0.600 | 243.40 | 5.255 | 26.333 | |
| 1_Dos | 13.556 | 20.869 | 0.840 | 90.700 | 5.020 | 11.942 | ||
| 2_Dos | 29.460 | 63.401 | 0.931 | 323.91 | 5.985 | 16.292 | ||
| Ferritin | Non-Vaccinated | 351.141 | 702.675 | 11.00 | 3000.0 | 93.595 | 167.85 | |
| 1_Dos | 102.837 | 151.601 | 6.99 | 724.00 | 67.335 | 56.54 | ||
| 2_Dos | 226.616 | 518.658 | 3.22 | 3000.0 | 78.450 | 112.11 | ||
Table 20: Descriptive of D-dimer, CRP and Ferritin according to vaccination levels
Table.21 shows that there are no significant differences in D-dimer, CRP and Ferritin according to the levels of vaccination, and vaccination has a weak effect upon D-dimer, CRP and Ferritin since ε2 less than0.04.
| Parameters | Vaccinated | N | Mean Rank | Kruskal-Wallis H | p-value |
| D- Dimer | Non | 44 | 85.28 | 4.017 | .134 |
| 1_Dos | 28 | 64.91 | |||
| 2_Dos | 78 | 73.78 | |||
| CRP | Non | 44 | 76.95 | .450 | .799 |
| 1_Dos | 28 | 70.55 | |||
| 2_Dos | 78 | 76.46 | |||
Ferritin | Non | 44 | 81.63 | 1.826 | .401 |
| 1_Dos | 28 | 67.55 | |||
| 2_Dos | 78 | 74.90 |
Table 21: The significancy of differences in D-dimer, CRP and Ferritin according to the levels of vaccination
Table.22 shows that there are statistically significant strong positive relations between; CRP and D-dimer with correlation coefficient r = 0.796, Ferritin and D-dimer with r = 0.712, CRP and Ferritin with r = 0.703. Furthermore, it shows that there are a statistically significant moderate relations between; RBC and HGB with r = 0.530, WBC and Ferritin with r = 0.515, WBC and
CRP with r = 0.503, WBC and D-dimer with r = 0.451, Granulocytes and CRP with r = 0.521, Granulocytes and D-dimer with r = 0.499, Granulocytes and Ferritin with r = 0.419. Again, there are statistically significant weak positive relations between; Granulocytes and WBC with r = 0.363, IgM and PLT with r = 0.306, IgM and CRP with r = 0.265, PLT and WBC with r = 0.187, Lymphocytes and HGB with r = 0.165. Moreover, there are statistically significant weak negative relations between; HGB and D-dimer with r = -0.435, HGB and Ferritin with r = -0.381, HGB and Granulocytes with r = -0.349, HGB and CRP with r = -0.339, HGB and WBC with r = -0.279, Again between Lymphocytes and Granulocytes with r = -0.411, Lymphocytes and CRP with r = -0.268, Lymphocytes and D-dimer with r = -0.263, Lymphocytes and ferritin with r = -0.233, finally, between RBC and Granulocytes with r = -0.244.
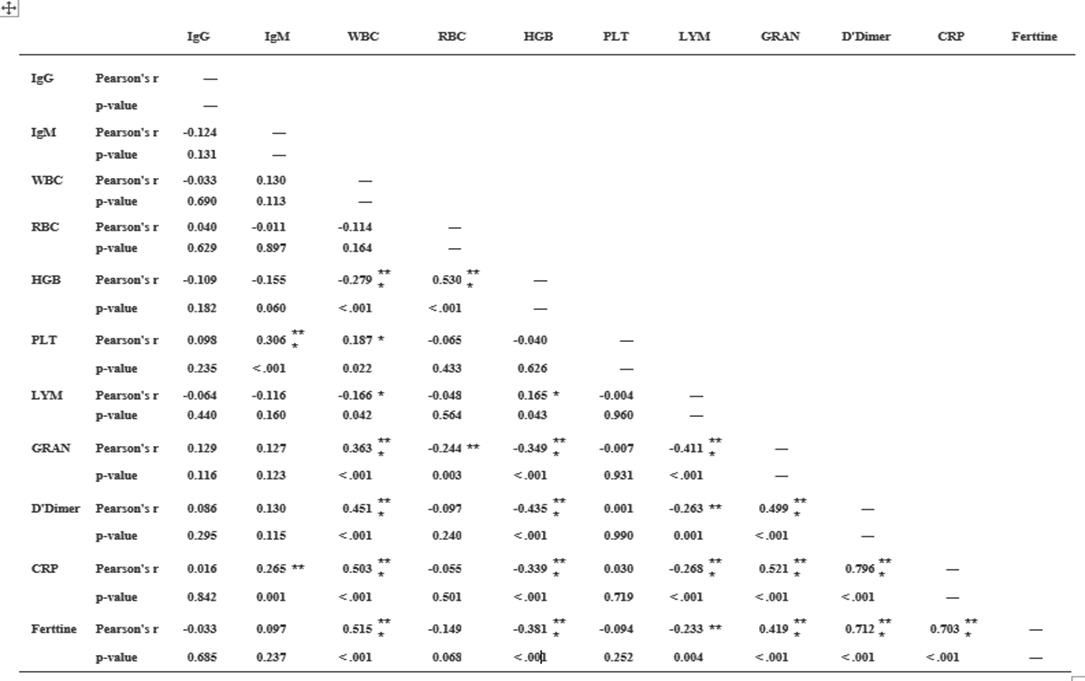
Table.22: Correlation Matrix
The current study showed that there was a statistically significant variation in the hematological parameters of COVID-19 patients between the 1st weeks (infection week) and 2nd week (a peak week). The current study demonstrated that there was decrease in lymphocytes and RBCs count that might be due to inflammatory responses, and these changes expanded as the disease progresses. Lymphocytopenia is frequent in patients with COVID- 19, which indicates a decadence of immunity during COVID-19 infection. It is observed that the decrease of lymphocytes was below the normal range in most infected patients; this concurs with the Gao et al. (19), and Zhou et al. (20) results.
On the other hand, there were no statistically significant (P>0.05) changes observed in the, hemoglobin concentration, WBCs, granulocytes, and platelets counts. Furthermore, Guan et al.(21), study showed low thrombocyte and leukocyte. Another study by Assiri et al., (22) and Xu et al. (23), noted thrombocytopenia in the patients and leukopenia in a different study. In addition, a study reported that thrombocytes decrease significantly in pneumonia patients and this reduction is proportionate with the clinical case of the patient. Several potential reasons have been suggested for thrombocytopenia in coronavirus patients as failure in thrombocyte production from classic cytokine storm in infection or attacking directly on hematopoietic stem cells, high destruction of platelet in circulating blood and decreased peripheral PLT secondary to lung damage (24). In the Chinese population, Duarte et al. (25), and Tan et al. (26), studies have reported the presence of leucopenia on hospital admission, basically at the expense of moderate to severe lymphopenia and mild thrombocytopenia, as well as a
decrease in hemoglobin, absolute monocyte counts and even tend to develop neutrophilia during hospitalization, with a peak in this period of ICU stay. Analysis of the baseline CBC parameters of the study population showed that 4 cases (12.9%) showed neutrophilia, 3(9.6%) cases showed lymphopenia, and 5 cases (16.1%) showed monocytosis. However, the baseline total leucocyte count was not increased (27). In contrast to the other studies conducted in China, whereby 63% of cases showed lymphopenia and 42
It can be concluded that coronavirus infection caused a significant decrease in lymphocytes, and RBCs count However, there were no statistically significant (P>0.05) changes observed in the, hemoglobin concentration, WBCs, granulocytes, and platelets counts in comparison to the healthy individuals. Also, COVID-19 caused a significant increase in IgM, IgG, D-dimer, CRP, and Ferritin levels at different periods compared to the controls. Further studies are needed to confirm these results. COVID-19 Specific Immunoglobulin's and Some hematological variables and Inflammatory factors in COVID-19 Patients These changes in IgM, IgG, D-dimer, CRP, and Ferritin levels during COVID-19 Virus Infection among COVID-19 patients may help the clinicians to better understand the COVID-19 and provide more clinical treatment options.
1 -Ministry of Health should develop an infectious disease preparedness and response plan that can help guide protective actions against COVID-19.
2 - Vaccination programs should be implemented including targeted to all people especially individuals with chronic diseases and pregnancy women, through all media and channels for spreading the needed Information.
3 – More studies should be conducted in order to have knowledge about the behavior of the new virus (COVID-19).
Clearly Auctoresonline and particularly Psychology and Mental Health Care Journal is dedicated to improving health care services for individuals and populations. The editorial boards' ability to efficiently recognize and share the global importance of health literacy with a variety of stakeholders. Auctoresonline publishing platform can be used to facilitate of optimal client-based services and should be added to health care professionals' repertoire of evidence-based health care resources.

Journal of Clinical Cardiology and Cardiovascular Intervention The submission and review process was adequate. However I think that the publication total value should have been enlightened in early fases. Thank you for all.

Journal of Women Health Care and Issues By the present mail, I want to say thank to you and tour colleagues for facilitating my published article. Specially thank you for the peer review process, support from the editorial office. I appreciate positively the quality of your journal.
Journal of Clinical Research and Reports I would be very delighted to submit my testimonial regarding the reviewer board and the editorial office. The reviewer board were accurate and helpful regarding any modifications for my manuscript. And the editorial office were very helpful and supportive in contacting and monitoring with any update and offering help. It was my pleasure to contribute with your promising Journal and I am looking forward for more collaboration.

We would like to thank the Journal of Thoracic Disease and Cardiothoracic Surgery because of the services they provided us for our articles. The peer-review process was done in a very excellent time manner, and the opinions of the reviewers helped us to improve our manuscript further. The editorial office had an outstanding correspondence with us and guided us in many ways. During a hard time of the pandemic that is affecting every one of us tremendously, the editorial office helped us make everything easier for publishing scientific work. Hope for a more scientific relationship with your Journal.

The peer-review process which consisted high quality queries on the paper. I did answer six reviewers’ questions and comments before the paper was accepted. The support from the editorial office is excellent.

Journal of Neuroscience and Neurological Surgery. I had the experience of publishing a research article recently. The whole process was simple from submission to publication. The reviewers made specific and valuable recommendations and corrections that improved the quality of my publication. I strongly recommend this Journal.

Dr. Katarzyna Byczkowska My testimonial covering: "The peer review process is quick and effective. The support from the editorial office is very professional and friendly. Quality of the Clinical Cardiology and Cardiovascular Interventions is scientific and publishes ground-breaking research on cardiology that is useful for other professionals in the field.
Thank you most sincerely, with regard to the support you have given in relation to the reviewing process and the processing of my article entitled "Large Cell Neuroendocrine Carcinoma of The Prostate Gland: A Review and Update" for publication in your esteemed Journal, Journal of Cancer Research and Cellular Therapeutics". The editorial team has been very supportive.

Testimony of Journal of Clinical Otorhinolaryngology: work with your Reviews has been a educational and constructive experience. The editorial office were very helpful and supportive. It was a pleasure to contribute to your Journal.

Dr. Bernard Terkimbi Utoo, I am happy to publish my scientific work in Journal of Women Health Care and Issues (JWHCI). The manuscript submission was seamless and peer review process was top notch. I was amazed that 4 reviewers worked on the manuscript which made it a highly technical, standard and excellent quality paper. I appreciate the format and consideration for the APC as well as the speed of publication. It is my pleasure to continue with this scientific relationship with the esteem JWHCI.

This is an acknowledgment for peer reviewers, editorial board of Journal of Clinical Research and Reports. They show a lot of consideration for us as publishers for our research article “Evaluation of the different factors associated with side effects of COVID-19 vaccination on medical students, Mutah university, Al-Karak, Jordan”, in a very professional and easy way. This journal is one of outstanding medical journal.
Dear Hao Jiang, to Journal of Nutrition and Food Processing We greatly appreciate the efficient, professional and rapid processing of our paper by your team. If there is anything else we should do, please do not hesitate to let us know. On behalf of my co-authors, we would like to express our great appreciation to editor and reviewers.

As an author who has recently published in the journal "Brain and Neurological Disorders". I am delighted to provide a testimonial on the peer review process, editorial office support, and the overall quality of the journal. The peer review process at Brain and Neurological Disorders is rigorous and meticulous, ensuring that only high-quality, evidence-based research is published. The reviewers are experts in their fields, and their comments and suggestions were constructive and helped improve the quality of my manuscript. The review process was timely and efficient, with clear communication from the editorial office at each stage. The support from the editorial office was exceptional throughout the entire process. The editorial staff was responsive, professional, and always willing to help. They provided valuable guidance on formatting, structure, and ethical considerations, making the submission process seamless. Moreover, they kept me informed about the status of my manuscript and provided timely updates, which made the process less stressful. The journal Brain and Neurological Disorders is of the highest quality, with a strong focus on publishing cutting-edge research in the field of neurology. The articles published in this journal are well-researched, rigorously peer-reviewed, and written by experts in the field. The journal maintains high standards, ensuring that readers are provided with the most up-to-date and reliable information on brain and neurological disorders. In conclusion, I had a wonderful experience publishing in Brain and Neurological Disorders. The peer review process was thorough, the editorial office provided exceptional support, and the journal's quality is second to none. I would highly recommend this journal to any researcher working in the field of neurology and brain disorders.

Dear Agrippa Hilda, Journal of Neuroscience and Neurological Surgery, Editorial Coordinator, I trust this message finds you well. I want to extend my appreciation for considering my article for publication in your esteemed journal. I am pleased to provide a testimonial regarding the peer review process and the support received from your editorial office. The peer review process for my paper was carried out in a highly professional and thorough manner. The feedback and comments provided by the authors were constructive and very useful in improving the quality of the manuscript. This rigorous assessment process undoubtedly contributes to the high standards maintained by your journal.

International Journal of Clinical Case Reports and Reviews. I strongly recommend to consider submitting your work to this high-quality journal. The support and availability of the Editorial staff is outstanding and the review process was both efficient and rigorous.

Thank you very much for publishing my Research Article titled “Comparing Treatment Outcome Of Allergic Rhinitis Patients After Using Fluticasone Nasal Spray And Nasal Douching" in the Journal of Clinical Otorhinolaryngology. As Medical Professionals we are immensely benefited from study of various informative Articles and Papers published in this high quality Journal. I look forward to enriching my knowledge by regular study of the Journal and contribute my future work in the field of ENT through the Journal for use by the medical fraternity. The support from the Editorial office was excellent and very prompt. I also welcome the comments received from the readers of my Research Article.

Dear Erica Kelsey, Editorial Coordinator of Cancer Research and Cellular Therapeutics Our team is very satisfied with the processing of our paper by your journal. That was fast, efficient, rigorous, but without unnecessary complications. We appreciated the very short time between the submission of the paper and its publication on line on your site.

I am very glad to say that the peer review process is very successful and fast and support from the Editorial Office. Therefore, I would like to continue our scientific relationship for a long time. And I especially thank you for your kindly attention towards my article. Have a good day!

"We recently published an article entitled “Influence of beta-Cyclodextrins upon the Degradation of Carbofuran Derivatives under Alkaline Conditions" in the Journal of “Pesticides and Biofertilizers” to show that the cyclodextrins protect the carbamates increasing their half-life time in the presence of basic conditions This will be very helpful to understand carbofuran behaviour in the analytical, agro-environmental and food areas. We greatly appreciated the interaction with the editor and the editorial team; we were particularly well accompanied during the course of the revision process, since all various steps towards publication were short and without delay".

I would like to express my gratitude towards you process of article review and submission. I found this to be very fair and expedient. Your follow up has been excellent. I have many publications in national and international journal and your process has been one of the best so far. Keep up the great work.

We are grateful for this opportunity to provide a glowing recommendation to the Journal of Psychiatry and Psychotherapy. We found that the editorial team were very supportive, helpful, kept us abreast of timelines and over all very professional in nature. The peer review process was rigorous, efficient and constructive that really enhanced our article submission. The experience with this journal remains one of our best ever and we look forward to providing future submissions in the near future.

I am very pleased to serve as EBM of the journal, I hope many years of my experience in stem cells can help the journal from one way or another. As we know, stem cells hold great potential for regenerative medicine, which are mostly used to promote the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. I think Stem Cell Research and Therapeutics International is a great platform to publish and share the understanding towards the biology and translational or clinical application of stem cells.

I would like to give my testimony in the support I have got by the peer review process and to support the editorial office where they were of asset to support young author like me to be encouraged to publish their work in your respected journal and globalize and share knowledge across the globe. I really give my great gratitude to your journal and the peer review including the editorial office.

I am delighted to publish our manuscript entitled "A Perspective on Cocaine Induced Stroke - Its Mechanisms and Management" in the Journal of Neuroscience and Neurological Surgery. The peer review process, support from the editorial office, and quality of the journal are excellent. The manuscripts published are of high quality and of excellent scientific value. I recommend this journal very much to colleagues.

Dr.Tania Muñoz, My experience as researcher and author of a review article in The Journal Clinical Cardiology and Interventions has been very enriching and stimulating. The editorial team is excellent, performs its work with absolute responsibility and delivery. They are proactive, dynamic and receptive to all proposals. Supporting at all times the vast universe of authors who choose them as an option for publication. The team of review specialists, members of the editorial board, are brilliant professionals, with remarkable performance in medical research and scientific methodology. Together they form a frontline team that consolidates the JCCI as a magnificent option for the publication and review of high-level medical articles and broad collective interest. I am honored to be able to share my review article and open to receive all your comments.

“The peer review process of JPMHC is quick and effective. Authors are benefited by good and professional reviewers with huge experience in the field of psychology and mental health. The support from the editorial office is very professional. People to contact to are friendly and happy to help and assist any query authors might have. Quality of the Journal is scientific and publishes ground-breaking research on mental health that is useful for other professionals in the field”.

Dear editorial department: On behalf of our team, I hereby certify the reliability and superiority of the International Journal of Clinical Case Reports and Reviews in the peer review process, editorial support, and journal quality. Firstly, the peer review process of the International Journal of Clinical Case Reports and Reviews is rigorous, fair, transparent, fast, and of high quality. The editorial department invites experts from relevant fields as anonymous reviewers to review all submitted manuscripts. These experts have rich academic backgrounds and experience, and can accurately evaluate the academic quality, originality, and suitability of manuscripts. The editorial department is committed to ensuring the rigor of the peer review process, while also making every effort to ensure a fast review cycle to meet the needs of authors and the academic community. Secondly, the editorial team of the International Journal of Clinical Case Reports and Reviews is composed of a group of senior scholars and professionals with rich experience and professional knowledge in related fields. The editorial department is committed to assisting authors in improving their manuscripts, ensuring their academic accuracy, clarity, and completeness. Editors actively collaborate with authors, providing useful suggestions and feedback to promote the improvement and development of the manuscript. We believe that the support of the editorial department is one of the key factors in ensuring the quality of the journal. Finally, the International Journal of Clinical Case Reports and Reviews is renowned for its high- quality articles and strict academic standards. The editorial department is committed to publishing innovative and academically valuable research results to promote the development and progress of related fields. The International Journal of Clinical Case Reports and Reviews is reasonably priced and ensures excellent service and quality ratio, allowing authors to obtain high-level academic publishing opportunities in an affordable manner. I hereby solemnly declare that the International Journal of Clinical Case Reports and Reviews has a high level of credibility and superiority in terms of peer review process, editorial support, reasonable fees, and journal quality. Sincerely, Rui Tao.
